Home > Other safety-related topics > B-cell Kinetics
B-cell Kinetics
|
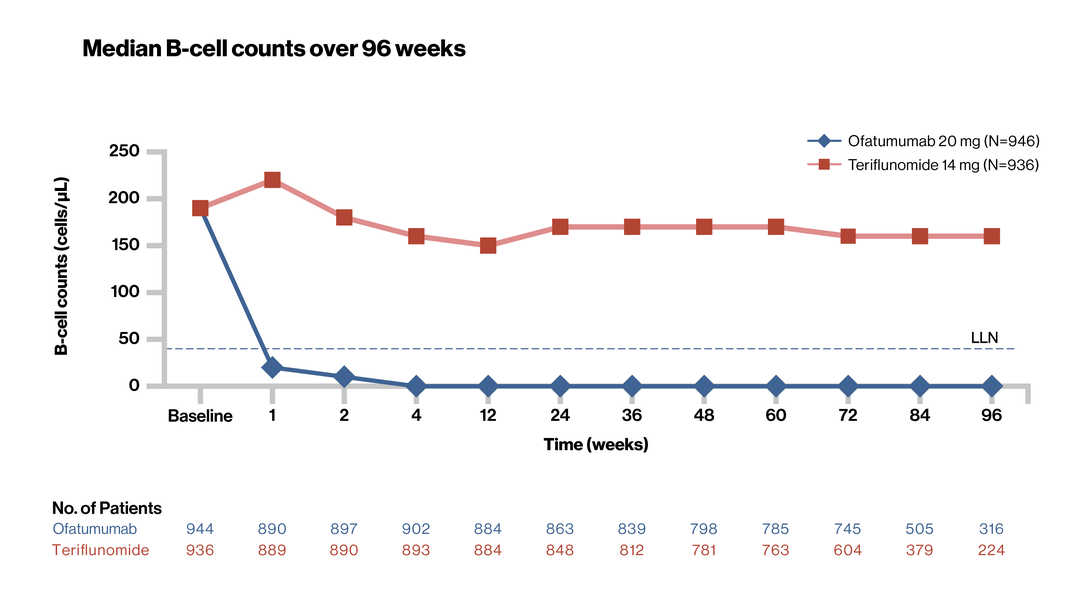
B-Cell Depletion and Efficacy Outcomes With Ofatumumab: Subgroup Analysis From the Pooled Phase 3 ASCLEPIOS I and II Trials. Hauser SL et al. AAN 2020.P7.1-013 |
|---|
|
Ofatumumab rapidly lowered B cells during the loading regimen (20 mg SC on Days 1, 7 and 14) in all patients regardless of body weight, and maintained low levels with ongoing monthly 20 mg SC injections |
SC, subcutaneous

Additional information

|
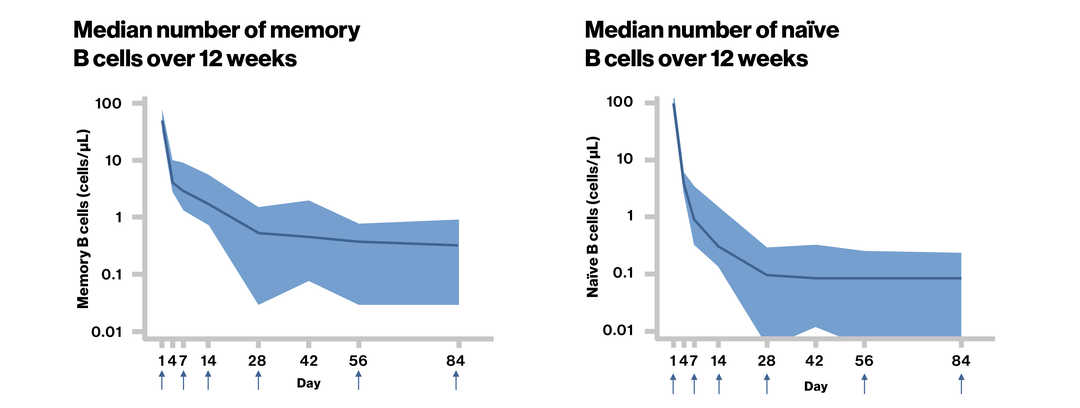
Effect of Subcutaneous Ofatumumab on Lymphocyte Subsets in Patients With RMS: Analysis from the APLIOS Study. Wiendl H, et al. EAN 2020. LB129.
|
|---|
|
Ofatumumab 20 mg SC led to rapid and sustained depletion of both total CD20+ B cells and CD20+ T cells in RMS patients in the APLIOS study |
↑ Dose administration; RMS, relapsing multiple sclerosis; SC, subcutaneous

|
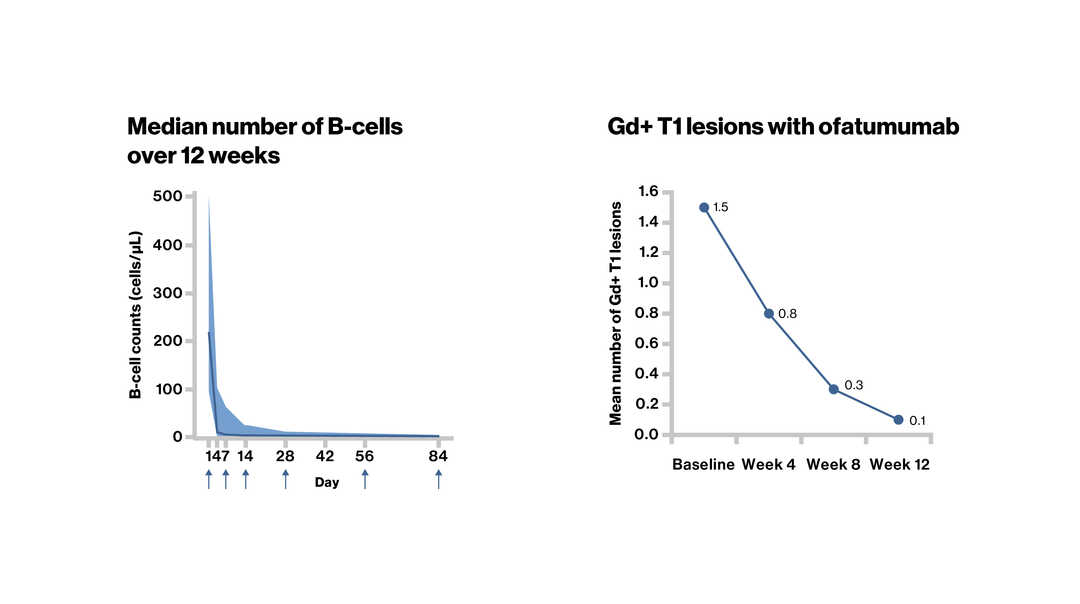
Onset of B-Cell Depletion and Suppression of MRI Activity With Ofatumumab Treatment in Relapsing Multiple Sclerosis: The APLIOS Study. Bar-Or A, et al. AAN 2020. P8.1-001.
|
|---|
|
Ofatumumab showed a rapid, close to complete, and sustained B-cell depletion, with no B-cell reconstitution. This corresponded to significant reduction of Gd+ lesions in RMS patients over 12 weeks |
↑ Dose administration; Gd+, gadolinium-enhancing; RMS, relapsing multiple sclerosis

|
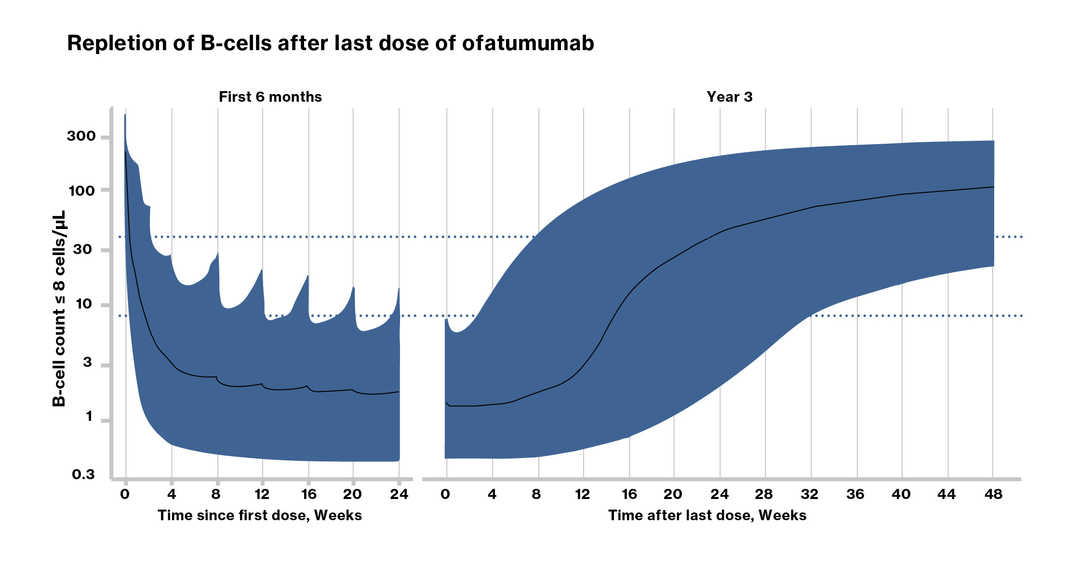
Population Pharmacokinetic-B Cell Modeling for Ofatumumab in Patients with Relapsing Multiple Sclerosis Yu H, et al. CNS Drugs . 2022 Mar;36(3):283-300
|
|---|
|
Data from RMS Phase 3 clinical studies indicate a median time to B-cell recovery to LLN or baseline value of 24.6 weeks post treatment discontinuation (Ref. EUPI) Pharmacokinetic (PK) B cell modelling and simulation for B-cell repletion corroborate this data, predicting median time to B cell recovery to LLN of 23 weeks post treatment discontinuation |
LLN, lower limit of normal;
*majority of B-cell repletion data are from ASCLEPIOS safety follow-up period

