Home > Other safety-related topics > Tolerability
Tolerability
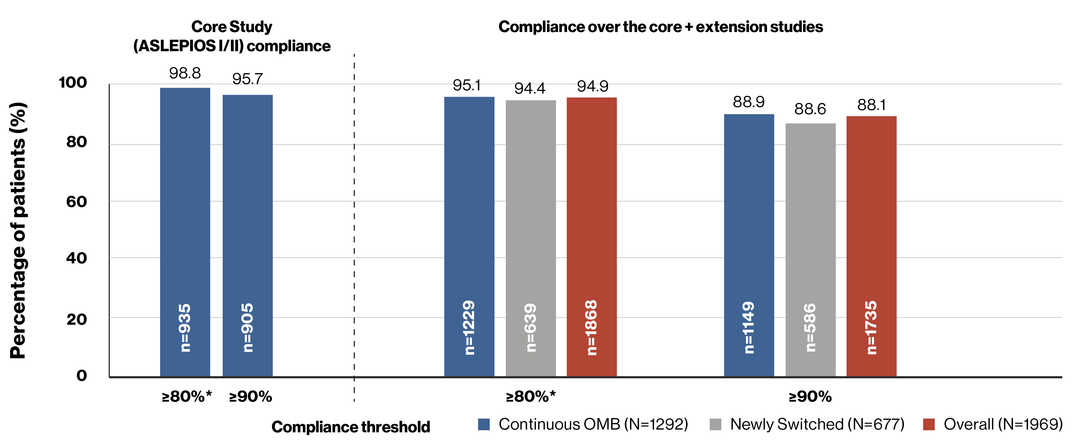
Compliance in ASCLEPIOS I/II (core) and ALITHIOS (extension) studies
|
Compliance and Persistence to Ofatumumab Treatment in Patients With Relapsing Multiple Sclerosis in Clinical Trials for Up to 4 years (25-Sep-2021) Alvarez E, et al. AAN 2023.
|
|---|
|
On-treatment period includes days from the first injection date until 30 days after the last injection date
*≥80% was defined as the threshold indicating a patient was compliant
n, number of patients; N, the number of patients entering the core ASCLEPIOS I/II trial and open-label ALITHIOS extension trial respectively; OMB, ofatumumab;

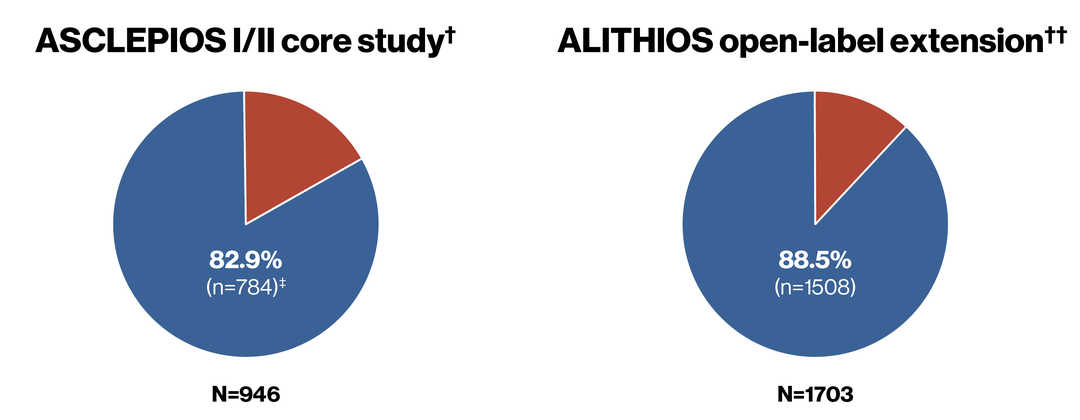
Persistence in ASCLEPIOS I/II (core) and ALITHIOS (extension) studies*
|
Compliance and Persistence to Ofatumumab Treatment in Patients With Relapsing Multiple Sclerosis in Clinical Trials for Up to 4 years. Alvarez E, et al. AAN 2023.
|
|---|
|
*Due to study duration differences between the ASCLEPIOS I/II (up to 30 months), APLIOS (12 weeks) and APOLITOS (24 weeks) core studies, persistence was calculated for the pooled ASCLEPIOS I/II core studies only. In addition, patients who successfully completed the core studies could opt not to continue onto the extension study. Therefore, persistence calculations were restricted to each study epoch to ensure patients who were persistent in the core studies but did not enter the extension did not confound persistence in the overall population analysis; ǂ One patient who completed OMB but had an end-of-study status as discontinued was not counted. †Persistence in patients randomised to OMB in the core study; ††Persistence in patients entering ALITHIOS up to the cut-off date of 25th-September-2021
OMB, ofatumumab.
