Home > Key safety topics > Malignancies
Malignancies in Clinical Trials
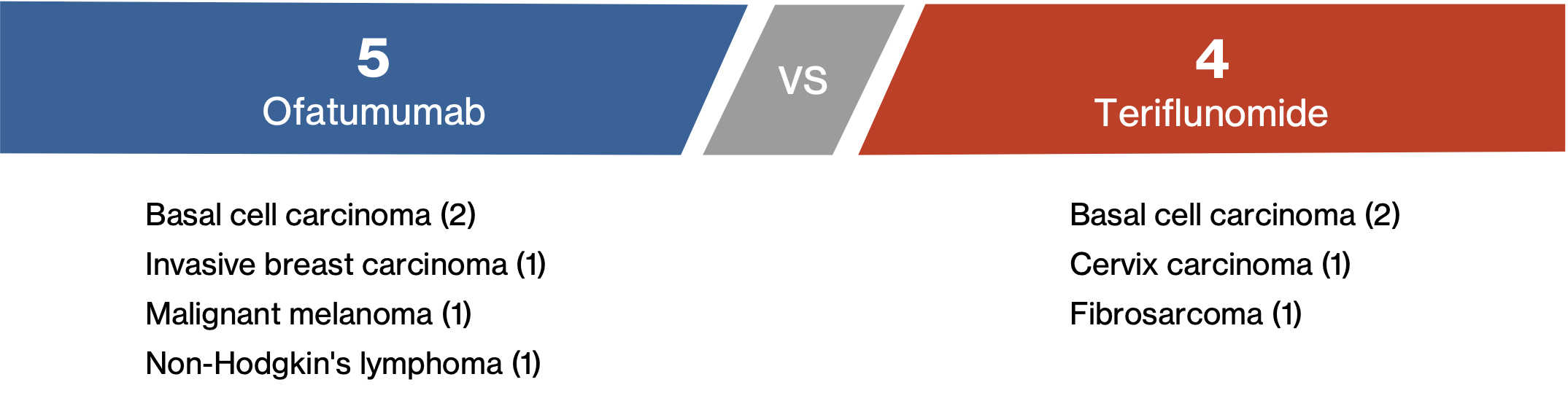
Controlled period1,a
- Five patients (0.5%) in the ofatumumab group reported malignant events compared to four patients in teriflunomide group (0.4%)
- Of the 5 patients in the ofatumumab group:
- Invasive breast carcinoma was reported within 5 months of treatment initiation
- Malignant melanoma in situ was reported within 39 days
- Non-Hodgkin's lymphoma (recurrent) was diagnosed within 31 days
- The two cases of basal cell carcinoma were confounded and assessed by the investigator as not related to ofatumumab therapy
- The four cases of basal cell carcinoma (two in each treatment group) resolved after surgery, and patients were allowed to remain on study treatment after full excision of basal cell carcinoma
OMB overall pool2,b
- As of 25-Sep-2023, EAIRs for malignancies did not increase over time in the overall ofatumumab population
for up to 6 years - Cumulatively (core+extension), malignancies were reported in 26 participantsc (1.32%) with EAIR of 0.32 (95% CI: 0.22–0.48)
- Median onset time since the first dose of ofatumumab was 843.5 days (31d–1931 days)
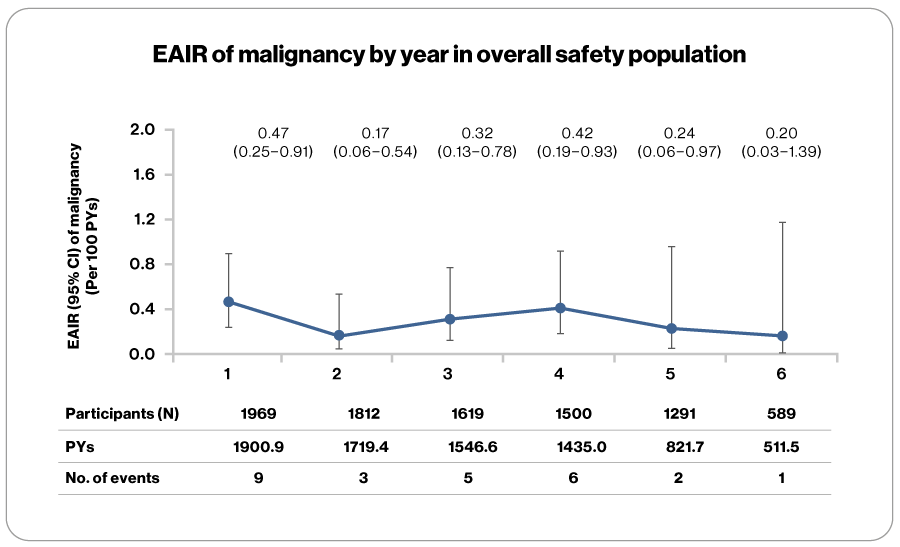
589 is the number of patients at-risk at least for some time during the 6th year, i.e., after year 5, where time started from first dose of OMB.
aincludes data of patients pooled from ASCLEPIOS I and ASCLEPIOS II trials during controlled treatment period.
bincludes cumulative data of all patients (N=1969) who were randomized to ofatumumab 20 mg in ASCLEPIOS I, ASCLEPIOS II, APLIOS and APOLITOS studies, completed the core period of the study, and continued to be treated with ofatumumab 20 mg in open-label ALITHIOS or either completed or discontinued the core period of the study and continued with safety follow-up epoch of the core study and newly switched group (who were randomized to teriflunomide 14 mg in ASCLEPIOS I and ASCLEPIOS II, completed the core period of the study, and switched to ofatumumab 20 mg in open-label ALITHIOS) until 25-Sep 2023 cut off.
cAnother case of non-serious malignancy (squamous cell carcinoma) was reported. dOne event with time-to-onset of 31 days was Non-Hodgkin's lymphoma recurrent. The 27 malignancies cases include breast and nipple neoplasms malignant (n=11), cervix neoplasm malignant (n=1), colorectal neoplasms malignant (n=1), metastases to specified sites (n=2), esophageal neoplasms malignant (n=2), neoplasms malignant site unspecified NEC (n=2), non-Hodgkin's lymphomas NEC (n=1), ovarian neoplasms malignant (excluding germ cell) (n=1), renal neoplasms malignant (n=2), skin melanomas (excluding ocular) (n=1), skin neoplasms malignant and unspecified (excluding melanoma) (n=5); n is the number of participants, and a participant can have >1 malignancy at a time.
Abbreviations
EAIR, exposure-adjusted incidence rate
References
1. Hauser SL, et al. N Engl J Med 2020;383:546−557.
2. Wiendl H, et al. Poster presented at AAN 2024. P9.010.