Home > Pivotal trials
Study Design
The ASCLEPIOS I and II trials had identical study design and were conducted in parallel1
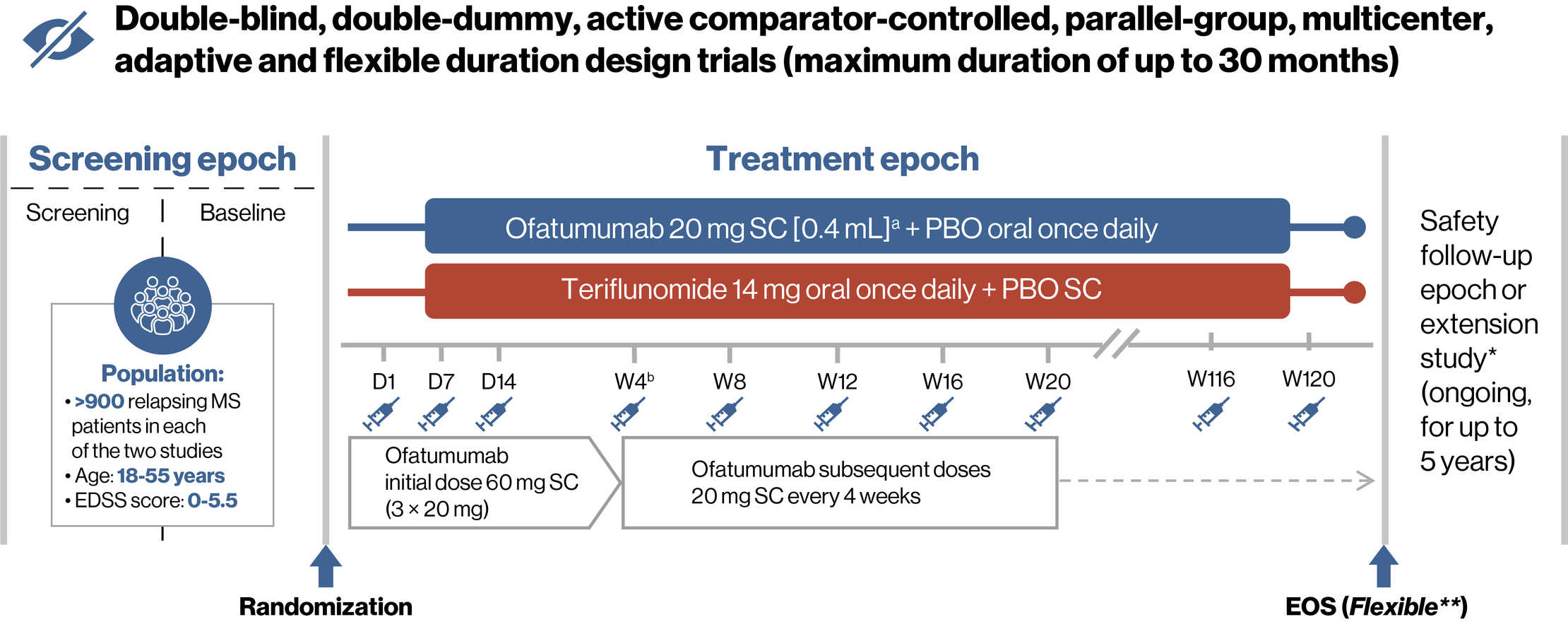
a20 mg of ofatumumab was administered in an injection volume of 0.4 mL; bWeek 4 (Month 1) and every 4 weeks thereafter.
*Open-label extension study (up to 5 years) via a separate protocol. Patients who complete the treatment epoch while on the study drug may be eligible to participate. The safety follow-up epoch is included to ensure all patients not entering the extension can have at least 9 months of follow-up after the last dose of the study drug.
**The end of study was projected based on a prospectively planned analysis of blinded data to provide 90% power for the primary endpoint, and 90 and 80% power for the 3- and 6-month confirmed disability worsening. EOS was defined by the amount of statistical information collected in the trial (relapses and disability events) instead of relying on a fixed time after the last patient has been randomized.
D, day; EDSS, Expanded Disability Status Scale; EOS, end of study; MS, multiple sclerosis; PBO, placebo; SC, subcutaneous; W, week

Demographics and Disease Characteristics at Baseline
The ASCLEPIOS I and II populations are consistent and poolable2
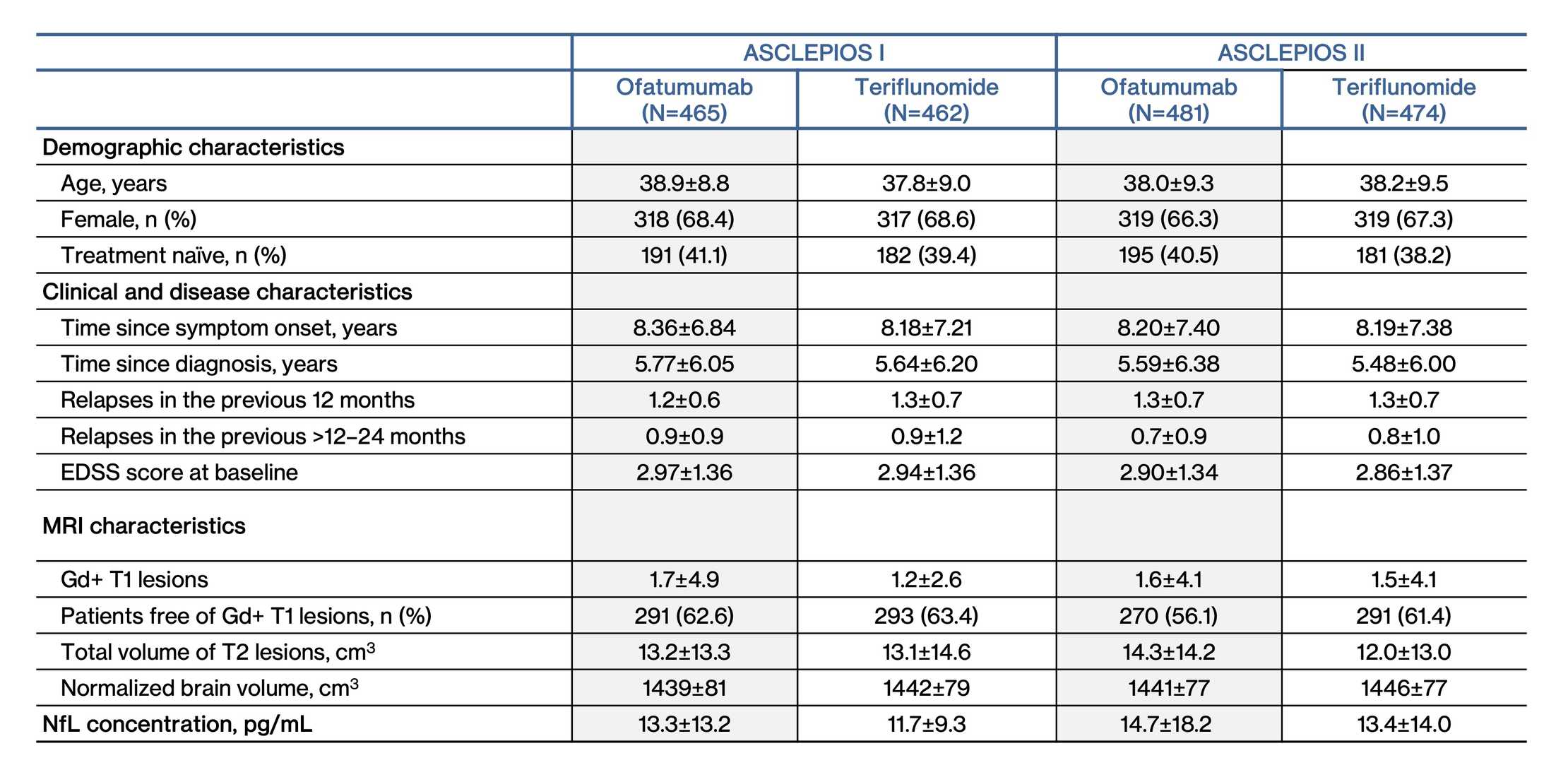
Data presented are mean±standard deviation unless otherwise specified
EDSS, Expanded Disability Status Scale; Gd+, gadolinium-enhancing; NBV, normalized brain volume; NfL, neurofilament light

Adverse Events
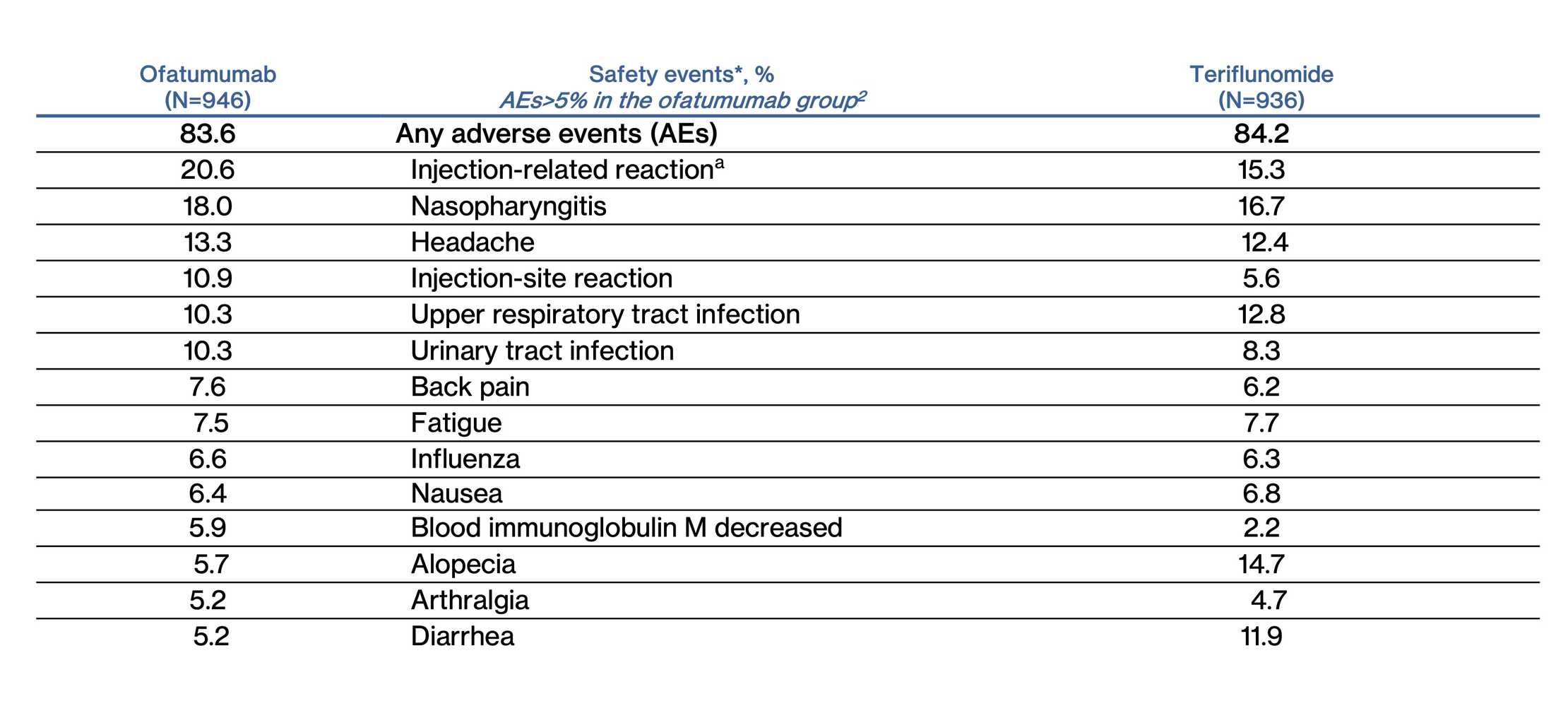
aInjection systemic reaction
*Includes adverse events reported in the pivotal trials which is different than adverse drug reaction in the US label. For details on adverse reactions, please refer to US PI
AEs are sorted in the order of frequency with ofatumumab

Injection-Related Reactions
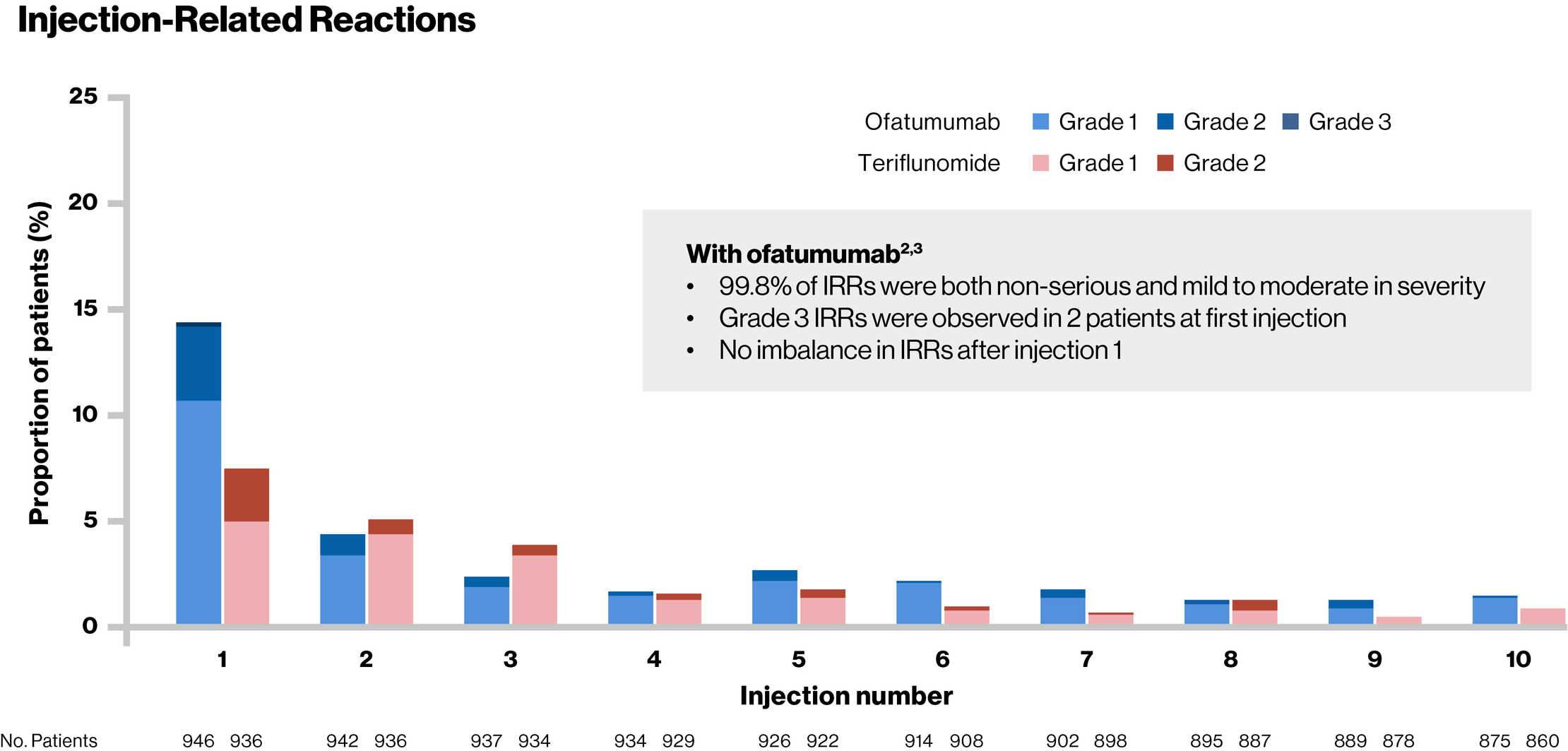
Patients in the teriflunomide group received matching placebo injections
IRR, injection-related reaction

Additional information
