1. What are the objectives of the ofatumumab safety website?
Novartis is fully committed to transparent and timely communication of safety updates associated with the use of ofatumumab in the treatment of multiple sclerosis. The primary objective of the safety website is to provide a digital resource that is easily accessible and has up-to-date and verified safety information for health care professionals (HCPs). Novartis will also continue to provide regular efficacy and safety updates through presentations at major congresses, in journal publications and other communication platforms.
2. How often is the website updated?
The full content of the website will be updated annually, according to the interim database lock of the long-term extension study (ALITHIOS). Additional updates may include important safety communications (e.g.: PML) and safety publications throughout the year. Reported COVID-19 cases to NVS will be updated XXXXXXXX (agreement still pending).
3. What are the limitations of the content of the website?
The safety website is not the primary source of efficacy and safety information for ofatumumab. In addition, safety data from the completed pivotal trials and the ongoing umbrella open-label extension trial to patients outside of the predefined trial criteria is limited. Owing to the inherent nature of the spontaneous and post-marketing reports, information may be incomplete and/ or duplicated. Novartis continuously verifies the information available on the website to ensure its accuracy. Therefore, the data are dynamic and subject to change. The local Prescribing Information/ Summary of Product Characteristics approved by individual countrys’ regulatory authority should be the primary source of product information used for ofatumumab.
4. Why is there information on only selected data/topics available on the website?
The scope of this website is to provide up-to-date information on the safety profile of ofatumumab. Based on the feedback from HCPs, experience from Novartis Multiple Sclerosis franchise, and criticality of certain adverse events, Novartis decided to select topics such as fatalities, malignancies, infections, COVID-19 and progressive multifocal leukoencephalopathy (PML) in patients who received ofatumumab for multiple sclerosis as the primary focus. Selection of these topics is not related to causation. Novartis is fully committed to transparent and timely communication of safety updates associated with ofatumumab in the treatment of RMS patients.
5. How do I get additional information on the safety of ofatumumab?
The local Prescribing Information/ Summary of Product Characteristics approved by individual country’s regulatory authority is the primary source of information related to safety and associated risks of treatment with ofatumumab in MS. For any additional questions on ofatumumab, you may reach out to the appropriate Novartis Medical Information associate in your country.
6. Are there differences in the reported rates of adverse events between this site and those on the FDA Adverse Event Reporting System (FAERS) database?
Novartis continuously monitors reporting of adverse events through a pharmacovigilance system. Novartis carefully assesses and evaluates all adverse events received and then submits a report to regulatory authorities as per requirements.
The FAERS database contains information on adverse events and medication error reports submitted to the FDA by HCPs, consumers, and manufacturers. It also includes case reports from other indications outside of MS. However, the information collected by the FAERS database is limited in scope/detail compared to Novartis pharmacovigilance procedures. This restricts the ability to rigorously evaluate an event and verify the causality of the event. Furthermore, duplicate reports can be submitted to the FAERS system, for example by both the sponsor and consumer. Therefore, FAERS data cannot be used to calculate the incidence of an adverse event, and rates of adverse events reported via the two systems can be expected to be different.
Ofatumumab related:
1. What is ofatumumab, and what is its proposed mechanism of action in MS?
Ofatumumab is a fully human monoclonal antibody (mAb) targeting the CD20 receptor. The CD20 is a cell surface antigen expressed on most B cell subsets and not expressed on plasma cells or pro B cells. Targeted depletion of CD20+ B cells has shown efficacy in reducing the inflammatory activity in MS.1
The precise mechanism by which ofatumumab exerts its therapeutic effects is unknown. It binds specifically to the CD20 molecule expressed on B cells (Figure 1).2, 3 In vitro data suggest that the binding of ofatumumab to the CD20 molecule leads to B-cell lysis via complement-dependent cytotoxicity (CDC) and antibody-dependent, cell-mediated cytotoxicity (ADCC).4
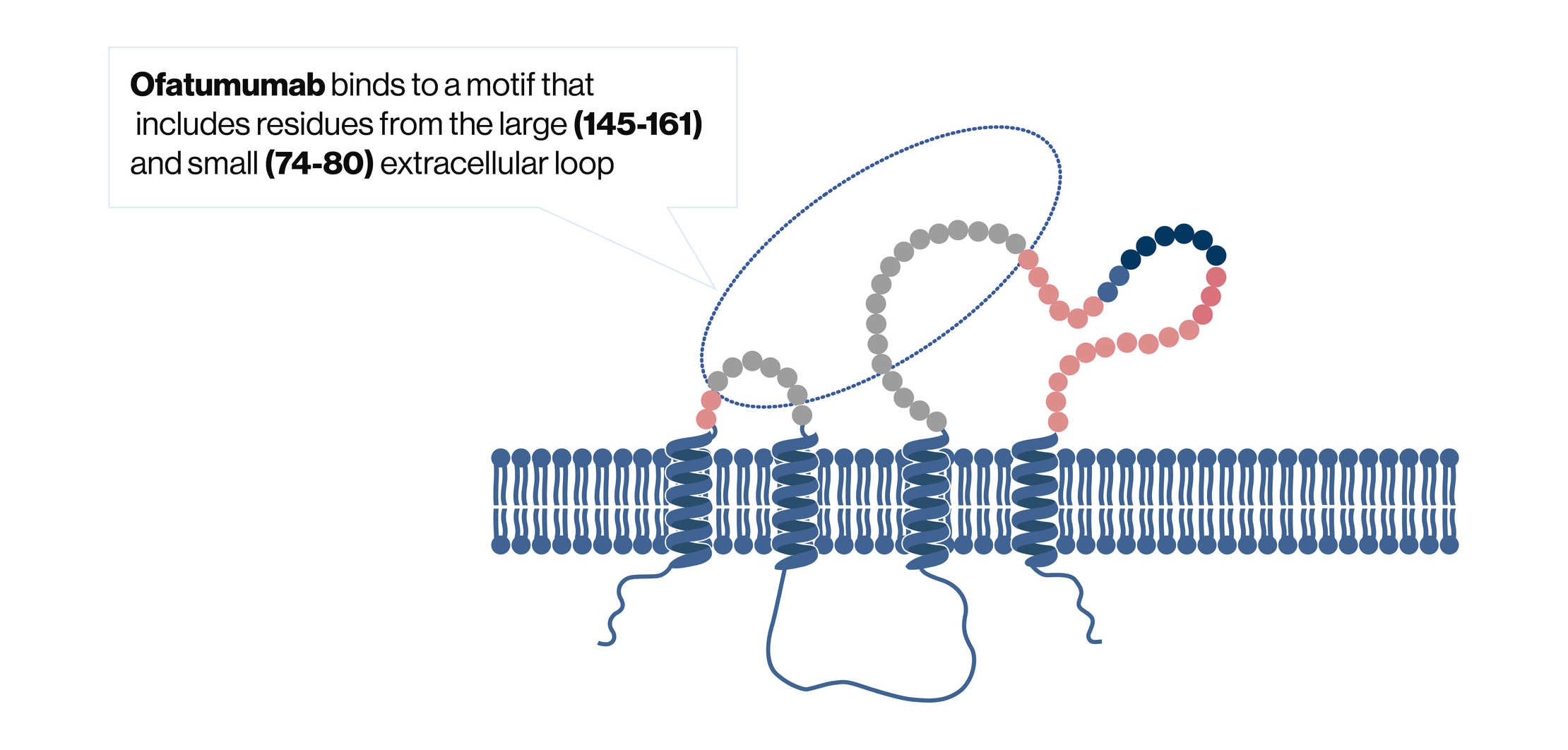
2. What is the rationale for subcutaneous route of administration?
Following the subcutaneous (SC) route of administration (Refer to the local Prescribing Information/ Summary of Product Characteristics approved by individual countrys’ regulatory authority for further details), monoclonal antibodies are predominantly absorbed via the lymphatic system.5, 6 Lymph nodes are the site of B cell-dependent T-cell activation through, for example, presentation of MS-relevant autoantigens by B cells to T cells. Therefore, specific targeting of lymph nodes might be achieved via the SC. route, and this may be relevant for MS disease pathology.7