Home > Key safety topics > Infections
Infection Rates in the MS Population

Participant Disposition in Ofatumumab Clinical Trials
- Controlled period: It includes 1882 participants from ASCLEPIOS I and ASCLEPIOS II trials.
- OMB overall pool: In the OMB overall pool, 86.5% patients (1703/1969) completed the core studies and entered ALITHIOS
- Of these, 78.1% (1330/1703) were still receiving ofatumumab at the time of cut off (25-Sep-2023)
- As of 25-Sep-2023, 416/1969 (21.1%) patients had at least 6 years (i.e., 288 weeks) of exposure to OMB from their first dose of OMB
Overall Infections in Ofatumumab Clinical Trials
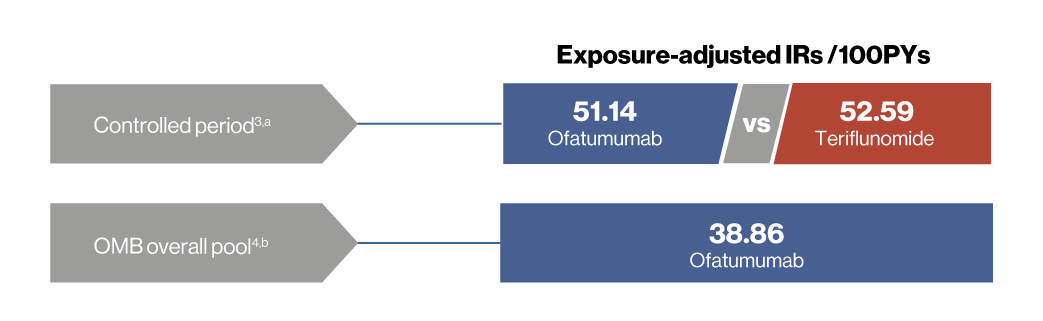
Controlled period3,a
- During the controlled period, exposure-adjusted IRs (EAIR/100 PYs) were similar in the ofatumumab (51.14) and teriflunomide (52.59) groups
- Most common infections were upper respiratory tract and urinary tract infections
- Herpes viral infections were reported similarly in both the groups i.e., ofatumumab (4.9%) and teriflunomide (4.2%). All herpes viral infections were non-serious, non-disseminated, resolved with treatment, and were not opportunistic
- Infections were mainly non-serious, Grade 1/2 severity, recovered with standard of care, and did not lead to study drug discontinuation or interruptions
OMB overall pool4,b
- As of 25 Sep 2023, EAIR per 100 PYs of infection over up to 6 years of ofatumumab treatment remained consistent with the ASCLEPIOS I/II trials
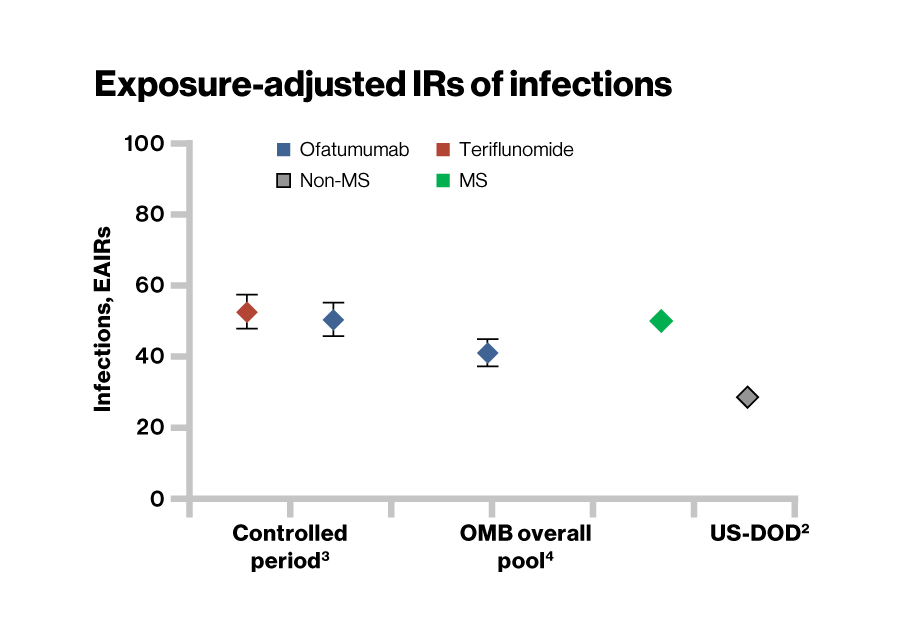
US-DOD: United states Department of Defense Study
Serious infections
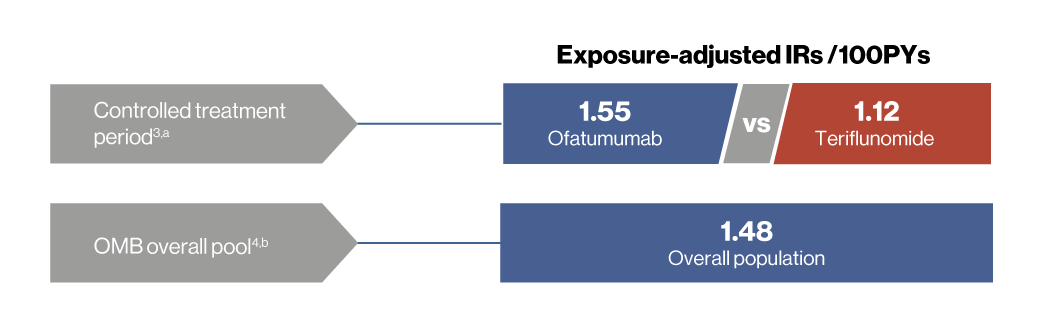
Controlled period3,a
- Overall, the incidence of serious infections was low in the ofatumumab and teriflunomide groups (2.5% and 1.8% of patients, respectively)
OMB overall pool4,b
- As of 25-Sep-2023, the overall EAIR per 100 PYs of serious infections was consistent with the ASCLEPIOS I/II trials (EAIR: 1.55) and no increased risk was observed for up to 6 years of ofatumumab treatment
- The most common serious infections were COVID-19 (1.4%)/COVID-19 pneumonia (1.3%)c and appendicitis (0.8%)d
Serious opportunistic infections
- As of 25-Sep-2023, one case of pneumocystis jirovecii pneumonia was reported in the long-term ofatumumab group. The final diagnosis was not confirmed by an external experte and the clinical course was not suggestive of pneumocystis jirovecii pneumonia4
- No cases of confirmed progressive multifocal leukoencephalopathy (PML) were reported in MS clinical studies or in the post-marketing setting with ofatumumab (see PML section for more details)
Serum immunoglobulin levels & risk of infections4
- Treatment effect of ofatumumab on IgG and IgM levels was analyzed for up to a period of 6 years
(Data cut-off: 25-Sep-2023) in the following groups:- Overall safety populationb (N=1969): Includes patients from Continuous ofatumumab and Newly switched groups
- Continuous ofatumumab (N=1292): Patients who were treated with ofatumumab in the core studies (ASCLEPIOS I/II, APLIOS, or APOLITOS), regardless of whether they entered ALITHIOS
- Newly switched group (N=677): Includes patients who received teriflunomide in the core part and switched to ofatumumab in the extension
- Overall safety populationb (N=1969): Includes patients from Continuous ofatumumab and Newly switched groups
- As of 25-Sep-2023, 416/1969 (21.1%) patients had at least 6 years (i.e., 288 weeks) of exposure to OMB from their first dose of OMB
- Serum immunoglobulin levels with ofatumumab treatment for up to 6 years (as of 25-Sep-2023), were consistent with the phase 3 ASCLEPIOS trial data, which showed that
- Mean serum IgG levels remained stable and above the LLN (5.65 g/L) throughout the entire treatment period in both groups. In the majority of patients (97.2%) receiving OMB for up to 6 years, IgG levels remained above the LLN at any time point4
- Mean IgM levels decreased over time but remained above the LLN (0.40 g/L) throughout the entire treatment period in both groups. In the majority of patients (65.9%) receiving OMB for up to 6 years, IgM levels remained above LLN at any time point4
- Treatment interruption/discontinuationf was reported in 3 (0.2%)/4 (0.2%) patients due to low IgG; and in 203 (10.3%)/71 (3.6%) patients due to low IgM4
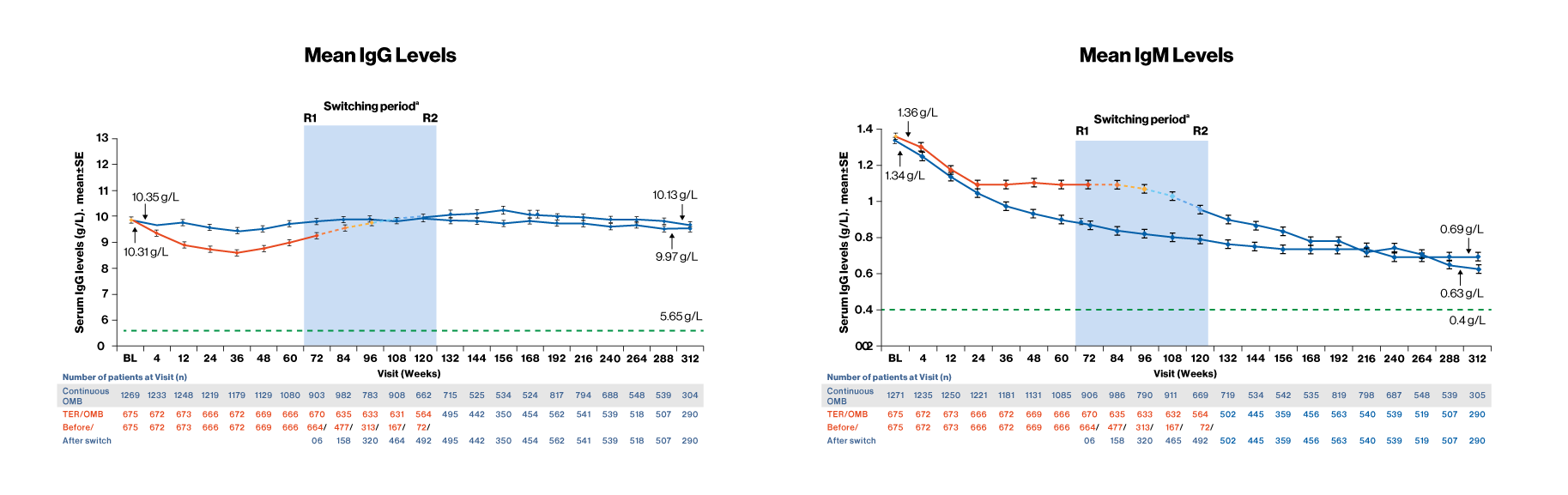
For all pooled analyses, a fixed value of LLN (using ALITHIOS study reference) was used: IgG: 5.65 g/L; IgM: 0.4 g/L at data cut off 25-Sep-2023. R1: The first patient with first treatment emergent assessment in OMB period after switching to OMB (72 weeks)
R2: The last patient with last treatment emergent assessment in TER period before switching to OMB (120 weeks)
aSwitching period refers to the participants started on teriflunomide and not applicable to the participants on ofatumumab in the core period. For the teriflunomide/ofatumumab group, data from the first dose of teriflunomide until the last dose of ofatumumab plus 100 days or analysis cutoff date has been used. R1: The first participant with first treatment-emergent assessment in ofatumumab period after switching to ofatumumab (72 weeks); R2: The last participant with last treatment-emergent assessment in teriflunomide period before switching to ofatumumab (120 weeks). For all pooled analyses, a fixed value of LLN (using ALITHIOS study reference) was used: IgG: 5.65 g/L and IgM: 0.4 g/L.
Ig, immunoglobulin; LLN, lower limit of normal; SE, standard error; RMS, relapsing multiple sclerosis; TER/OMB, switched from teriflunomide to ofatumumab.
For IgG levels, at year 6; 304 is the number of long-term patients with an IgG assessment at both baseline and Week 312, while 290 is the number of newly-switched patients with an assessment at both baseline and Week 312, where Week 312 for the switched group doesn’t mean that patients were on OMB for 312 weeks. For IgM levels, at year 6; 305 is the number of long-term patients with an IgG assessment at both baseline and Week 312, while 290 is the number of newly-switched patients with an assessment at both baseline and Week 312, where Week 312 for the switched group doesn’t mean that patients were on OMB for 312 weeks.
Additional information
Please refer to the below poster for details on sensitivity analysis for the Ig levels in patients with relapsing multiple sclerosis.


aIncludes data of patients pooled from ASCLEPIOS I and ASCLEPIOS II trials during controlled treatment period.
bIncludes cumulative data of all patients (N=1969) who were randomized to ofatumumab 20 mg in ASCLEPIOS I, ASCLEPIOS II, APLIOS and APOLITOS studies, completed the core period of the study, and continued to be treated with ofatumumab 20 mg in open-label ALITHIOS or either completed or discontinued the core period of the study and continued with safety follow-up epoch of the core study and newly switched group (who were randomized to teriflunomide 14 mg in ASCLEPIOS I and ASCLEPIOS II, completed the core period of the study, and switched to ofatumumab 20 mg in open-label ALITHIOS) until 25-Sep 2023 cut off.
cThere were 49 COVID-19–related SAEs in total; one of them has PT of “suspected COVID-19,” and a majority (85.71%) of the cases recovered.
dAll cases of appendicitis recovered, and a majority of them were not related to ofatumumab treatment. f Participant was suspected to have serious Grade 2 P. jirovecii pneumonia and was assessed by an independent external expert.
eall serious infections will be adjudicated by an external expert.
fPer core and extension study protocols, investigators were required to interrupt study treatment if IgM levels fell below 10% LLN or IgG levels fell below 20% LLN. The requirement to interrupt treatment due to low IgM or IgG levels was removed with protocol amendment 2 for study COMB157G2399 and is left to the discretion of the investigator; Treatment interruption Preferred Term due to low IgM include blood immunoglobulin M decreased, immunoglobulins decreased, hypogammaglobulinemia and hypoglobulinemia while for discontinuation includes blood immunoglobulin M decreased, immunoglobulins decreased, blood immunoglobulin M abnormal and hypogammaglobulinemia. Treatment interruption PT due to low IgG include blood immunoglobulin G decreased and for discontinuation include immunoglobulins decreased, blood immunoglobulin G abnormal, blood immunoglobulin G decreased