
aProspective cases are defined as cases for which, at the time of initial reporting (i.e., first receipt by Novartis), the pregnancy outcome has not yet occurred or there is no report of an abnormal prenatal testing result (including cases where prenatal testing has not yet been performed, or cases where prenatal testing has been performed but results were either normal or not specified
bRetrospective cases are defined as cases for which at the time of initial reporting (i.e., first receipt by Novartis), the pregnancy outcome has already occurred, or prenatal testing results were abnormal (regardless of whether the pregnancy outcome has occurred)
Prospective cases
- As of September 25, 2023, 279 prospective pregnancies with maternal exposure to ofatumumab were identified
- A total of 55 pregnancy outcomes were known in 279 prospectively reported pregnancies
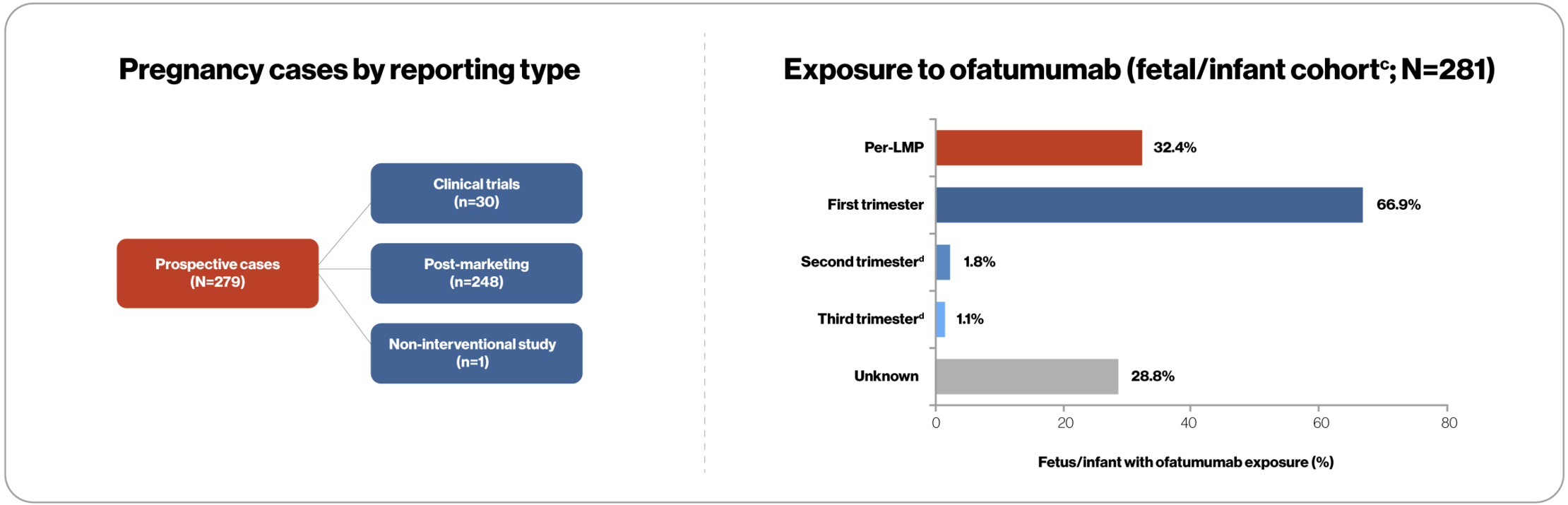
- The majority of in utero exposures (n=188; 66.9%) to ofatumumab occurred during the first trimester; for 81 patients (28.8%), the exact timing of exposure was unknown
- No major congenital anomalies or serious infections were observed
cThe 279 prospective pregnancy cases included a cohort of 281 fetuses/infants (two pregnancies involving twins). Some pregnancies involved more than one trimester of exposure to ofatumumab and therefore are included in more than one category in the figure above. The peri-LMP period for ofatumumab refers to the 180 days prior to the LMP. dCases exposed to ofatumumab in the second and third trimester either resulted in normal live births, or include pending outcomes or were lost to follow-up.
LMP, last menstrual period.
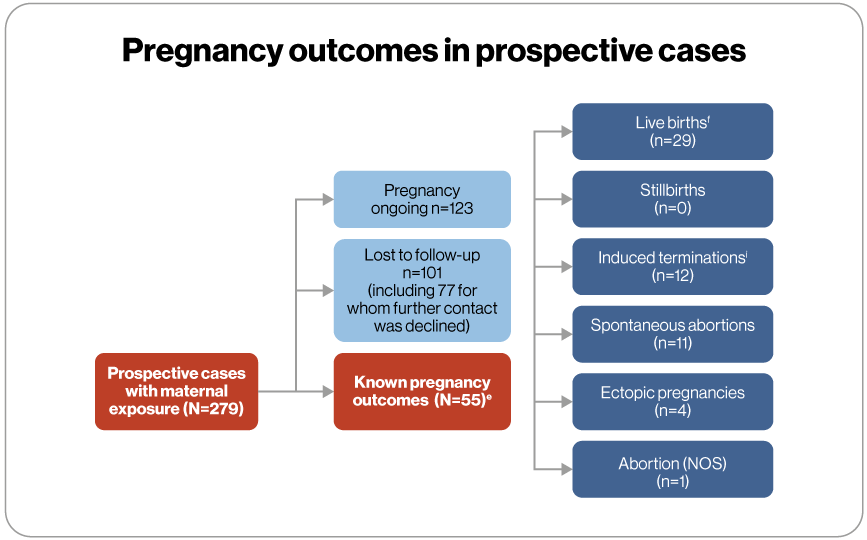
eTwo pregnancies involving twins. fIncludes newborn with a minor congenital malformation (hydronephrosis) and one set of twins. iIncludes therapeutic and elective terminations with another set of twins; One case of trisomy 18 and no reported abnormalities or reason for termination provided in the remaining 11 outcomes.
NOS, not otherwise specified.
Retrospective cases
- As of September 25, 2023, 30 retrospective pregnancy cases were reported in women with MS who were treated with ofatumumab. One patient discontinued therapy with ofatumumab due to delivery; no further details were provided
- Outcomes in the remaining 29 cases included 9 live births, 3 induced terminations, 16 spontaneous abortions, and 1 ectopic pregnancy
- No congenital anomalies were reported
| A prospective observational registry on maternal and infant outcomes in women exposed to ofatumumab during pregnancy is currently active. If pregnancy test shows positive, please contact the registry |
References
1. Bove R, et al. Poster presented at ACTRIMS 2024. P510.
2. Bove R, et al. Poster presented at AAN 2023.