Home > Key safety topics > COVID-19
In the ALITHIOS open-label extension trial1 (Link to the article)
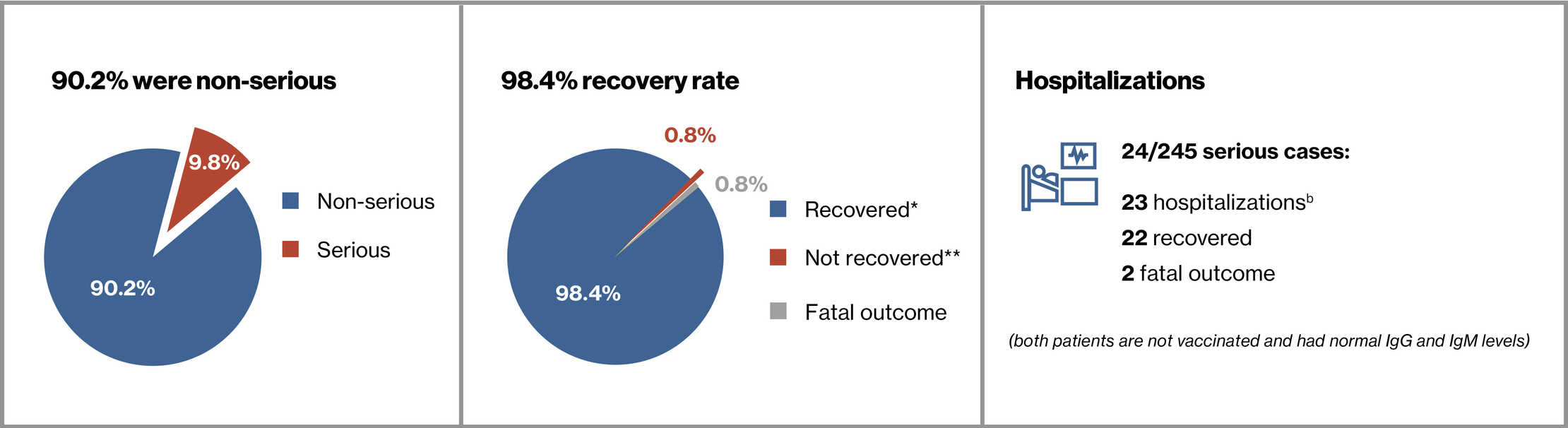
- As of 25 Sep 2021, 245 of 1703 RMS patients (14.4%) receiving ofatumumab in the ALITHIOS study reported COVID-19 (confirmed: 210 [85.7%]; suspected: 35 [14.3%])
- A total of 24 patients reported serious cases (23 were hospitalized and 2 patients required non-invasive mechanical ventilation)
- Two patients (0.8%) had fatal outcome
- No patient had reinfection
- Ofatumumab was temporarily interrupted in 39 (15.9%) patients
- Before COVID-19 onset, IgG levels were within the normal range in all COVID-19–affected patients, while IgM was
<0.4 g/L in 23 (9.4%) patients (22 out of these 23 were non-serious cases) - At the time of the cut-off, the majority (241, 98.4%) of COVID-19 cases were recovered or recovered with sequelae, or were recovering
- Baseline characteristics of COVID-19 cases in the overall and hospitalized cases are presented in table below
Baseline characteristics of COVID-19 cases
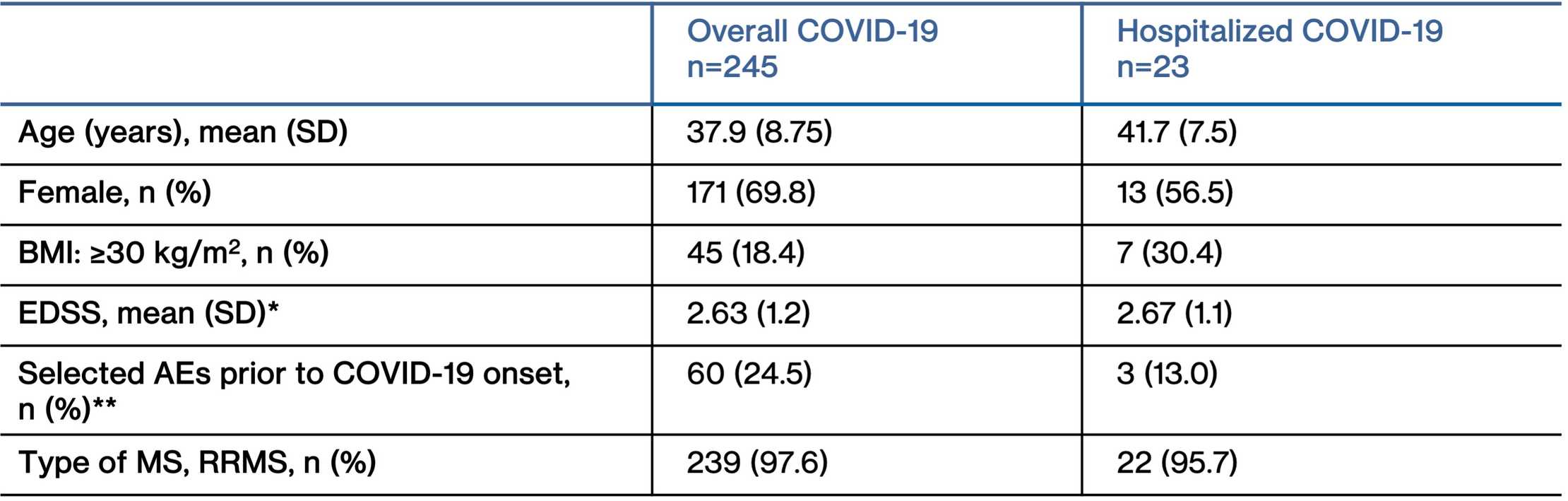
*EDSS at first dose of ofatumumab
**The selection of prior AEs was based on potential risk factors for more severe COVID-19 and included the following MedDRA System Organ Classes (SOCs) ‘Cardiac disorders,’ ‘Metabolism and nutrition disorders,’ ‘Respiratory, thoracic and mediastinal disorders,’ and ‘Vascular disorders.’
Summary of COVID-19 cases
|
Case 1: The first fatal COVID-19 case was a female patient in her 4th decade of life from Europe who was on ofatumumab for 3.7 years |
|
Case 2: The second fatal COVID-19 case was a male patient in his 3rd decade of life from Asia who was on ofatumumab treatment for 1.5 years. |
aseriousness category was based on the regulatory reporting definition established by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines E2A; bone of the 24 serious cases was not hospitalized due to personal circumstances and financial reasons and resulted in fatal outcome
*recovered includes recovered or recovered with sequalae or recovering at the time of data cutoff; **at the time of data cutoff
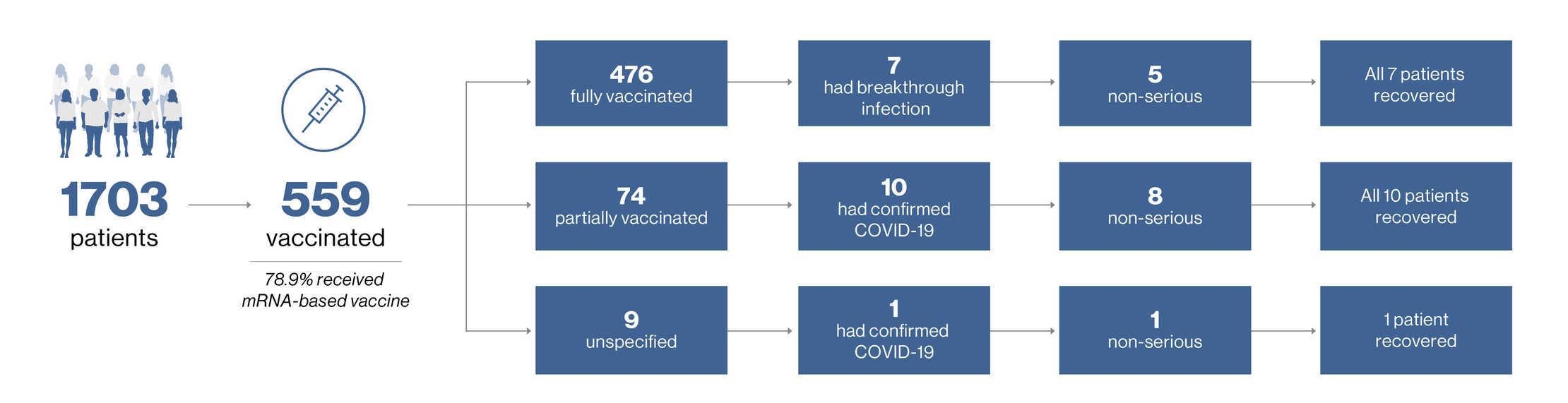
Vaccination and infections post vaccination as of 25-Sept-2021
Of the 559 patients with a COVID-19 vaccination, 37 (6.6%) had a confirmed ofatumumab dose interruption based upon physician decision and received a COVID-19 vaccination during the treatment gap2
In the post-marketing setting2
- The cumulative post-marketing patient exposure since the first launch of ofatumumab up to data cut off is estimated to be ~18,530 patient-treatment years.
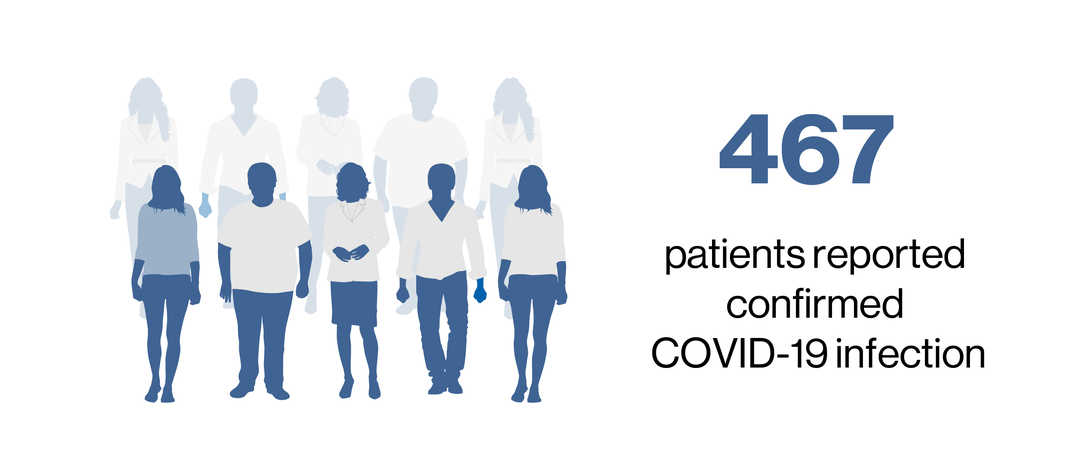
- As of 25 March 2022, 467 confirmedc and 13 suspectedd COVID-19 cases were reported to the Novartis Safety Database from the post-marketing setting in ofatumumab-treated patients
Summary of Confirmed Cases: N=90
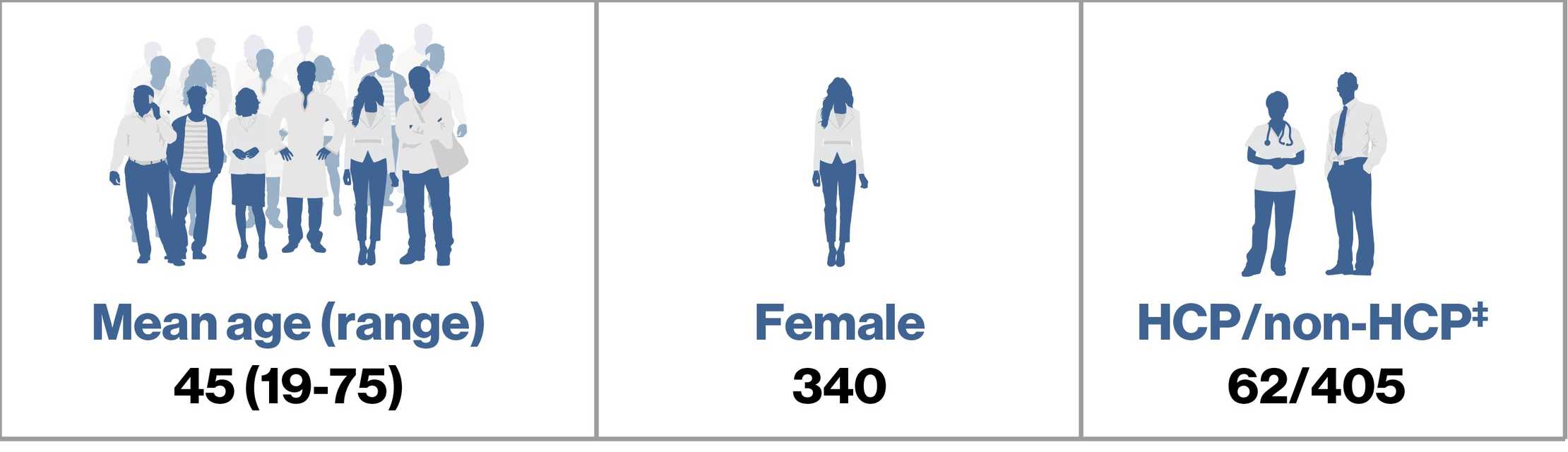
cif a SARS-CoV-2–positive test result was available or if the patient was reported to have been diagnosed with COVID-19;
dwithout a positive test result or a definitive diagnosis; ‡refers to who reported the case, HCP or non-HCP
HCP, healthcare professional.
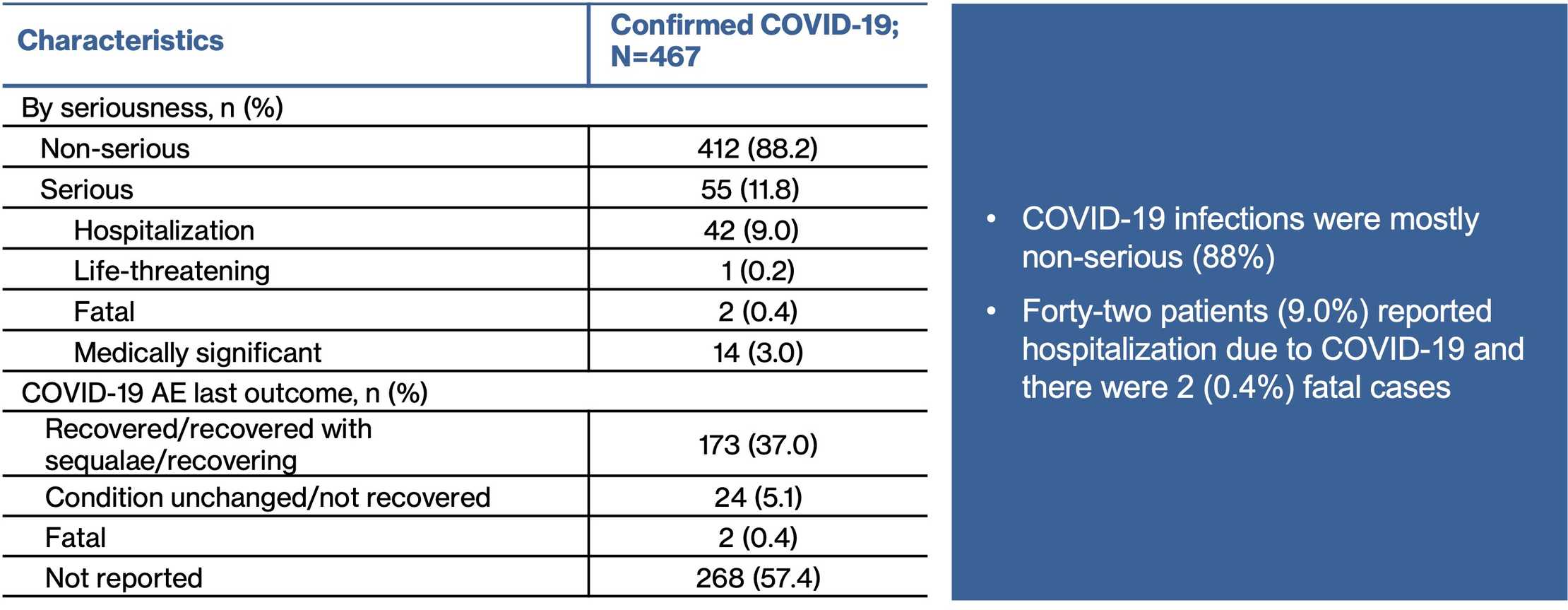
A case may have more than one seriousness criteria
AE, adverse event; HCP, healthcare professional.

Additional information





Immune response to COVID-19 vaccines in plwMS under ofatumumab treatment
Novartis is conducting/supporting various clinical studies to evaluate the effect of immune response to SARS-CoV-2 mRNA vaccination and other vaccines in MS patients treated with DMTs. Please see below for additional details
Additional information













Impact of COVID-19 in MS in the real-world setting
- MS is an autoimmune, chronic inflammatory, neurodegenerative disorder of CNS where patients are generally treated with immunosuppressants or immunomodulators.3 The current COVID-19 pandemic has raised concerns regarding the immune response to viral infections and post vaccination in MS patients treated with disease modifying therapies4
- Comprehensive data sharing and analyses regarding the effect of COVID-19 in people with MS have been conducted by the COVID-19 in MS - GDSI.5 The GDSI initiative is a joint initiative of the MS International Federation and the MS Data Alliance, acting under the umbrella of the European Charcot Foundation and in collaboration with many (data) partners across the globe
- In people with MS, the incidence of COVID-19 varies from 0.5% to 1.13%.6 The mortality due to COVID-19 has been reported from 1.6% to 4.2%6,7
- Prosperini L, et al. reported crude death rate of 1.97% and estimated indirectly-adjusted age-standardized lethality ratio to be 1.24 suggesting 24% increased risk of death from COVID-19 in patients with MS8. That excess of risk of mortality due to COVID-19 was confirmed by the Italian MusC-19 Study Group only for those patients who belonged to the ‘higher-risk- group' (EDSS > 3.0 or at least 1 comorbidity).9
Ofatumumab and COVID-19 – Guidance to HCPs
- Novartis is committed to patient's health and safety. In these unprecedented times, we are striving to keep patients, care partners, and healthcare providers up to date and provide the latest information to help inform decisions related to the use of our products. Novartis continues to collect data about COVID-19 infection in patients treated with ofatumumab.
- In general, patients and prescribers should act in accordance with local government and health authority guidance concerning the COVID-19 pandemic (including guidance on social distancing and self-isolation, as applicable). HCPs may also consult advice specific to patients with MS provided by international or local HCPs and patient organizations10-12 Ofatumumab has the potential for an increased risk of infections.3,13 Administration should be delayed in patients with an active infection until the infection is resolved.3,13 Novartis believes that treatment decisions should be made between a patient and their treating healthcare professional based on a benefit-risk assessment specific to the individual patient.
SARS-CoV-2 vaccination considerations
- To date all of the SARS-CoV-2 vaccines currently approved and available or in development belong to several categories/platforms, namely : (1) mRNA-based vaccines, (2) nonreplicating viral-vector vaccines, (3) inactivated vaccines, (4) protein vaccines and (5) live attenuated vaccines
- As with inactivated vaccines, the use of nonreplicating viral-vector vaccines or mRNA based SARS-CoV-2 vaccines in patients receiving immunomodulant/immunosuppressant therapies such as ofatumumab may have a diminished immune response
- Novartis is conducting several clinical studies to evaluate the humoral and cellular immune response post COVID-19 vaccination in people living with RMS receiving treatment with ofatumumab according to the regular clinical practice. Some preliminary results from their interim analysis are already available.14-16
- To date the available data of the occurrence and severity of breakthrough infections after a full course of vaccination in people living with MS (plwMS) is very scarce. Its frequency varies from 2% for Delta variant to 6% for Omicron variant. 17,18
- There is presently no contraindication for the use of inactivated, nonreplicating viral-vector, or mRNA-based SARS-CoV-2 vaccines while on treatment with ofatumumab, even if vaccinations may be less effective3,13
- Vaccination against SARS-CoV-2 should be considered on a case-by-case basis at the discretion of the treating physician and should be in adherence to immunization guidelines in the local vaccine label19
- Please review local prescribing information for any specific SARS-CoV-2 vaccine and comply with local prescribing information requirements for specific contraindications and special warnings and precautions for use
- People with MS who are considered to be immunocompromised could be advised to receive an additional dose of COVID-19 vaccine depending on local recommendations of their countries20. In some countries some other prophylaxis treatment could also be available as add-on strategy to minimize the risk of getting infected by SARS-CoV-2.21
- All immunizations should be administered according to local immunization guidelines, at least 4 weeks prior to initiation of ofatumumab for live or live-attenuated vaccines and, whenever possible, at least 2 weeks prior to initiation of ofatumumab for inactivated vaccines3,13
- The safety of immunization with live or live-attenuated vaccines following ofatumumab therapy has not been studied. Vaccination with live or live-attenuated vaccines is not recommended during treatment and after discontinuation until B-cell repletion3,13
- The guidance provided by National Multiple Sclerosis Society (NMSS) suggests that people with MS who are fully vaccinated with an mRNA vaccine and using S1PR modulators, alemtuzumab, and anti-CD20 therapies may benefit from an additional dose of mRNA vaccine and from the use of Evusheld* (tixagevimab co-packaged with cilgavimab)21,22
- Regarding the use of REGEN-COV* (casirivimab and imdevimab, administered together) as post-exposure prophylaxis (preventative) for COVID-19, the NMSS said that is safe to use with MS disease modifying therapies (DMTs), but the timing might need to be coordinated.23
- Some other countries and regions are also recommended to go for additional dose of vaccine after being fully vaccinated in people with immunosuppressive conditions (due to their disease or their treatment)24-26 and they are also considered some pre- and post-exposure prophylaxis* agents to be used in some people on higher risk of more severe course of COVID-19 (plwMS could be included in).27
*All pre and post-exposure prophylaxis should be administered according to local guidelines and the corresponding product information.

FAQs
1. Where do I find safety information related to COVID-19 infections with ofatumumab treatment?
- Novartis is committed to proactive and transparent safety communications of data related to COVID-19 infections with the MS community regularly in the coming months (e.g., local label, publications and website)
- Please refer to the ofatumumab Prescribing Information for relevant information regarding the risk of infections.
2. Should RMS patients initiate treatment with ofatumumab?
- Prior to initiating therapy, follow local guidelines on testing for SARS-CoV2. Patients can initiate therapy with ofatumumab in accordance with Prescribing Information approved by your national regulatory authorities.
3. Are patients on therapy with ofatumumab at a higher risk for COVID-19 infection?
- Based on its mode of action of depleting B cells, ofatumumab has the potential for an increased risk of infections, including COVID-19. Patients should consult with their physicians and treatment decisions should be made based on individual patient benefit-risk assessment. Please refer to the Prescribing Information for information regarding the safety of ofatumumab
4. Does treatment with ofatumumab cause a more severe course of SARS CoV-2 infection?
- There is no consistent evidence that MS patients treated with B-cell targeting therapies are at a higher risk for a more severe course of infections. Although, most of the registries currently on going reported a higher risk of having a more severe course of COVID-19 in plwMS exposed to RTX or OCR (including hospitalization but not death), the available information for ofatumumab in these registries is nearly zero. Among plwMS, risk factors for a more severe course of infection seem to be higher EDSS score, recent use of corticoids, progressive MS phenotype, obesity, age, and comorbidities. 5-9,28-32
- Our clinical data suggest that COVID-19 follows a similar course of SARS CoV-2 infection in ofatumumab-treated patients as in the general population1
- Two cases with COVID-19 had fatal outcome. Neither of these cases were vaccinated against COVID-19. Their serum IgG and IgM levels were within the normal range throughout the study1
- There has been a report of one COVID-19 pneumonia fatality in clinical trial. The investigator considered the event as not related to treatment with ofatumumab1
- The known benefit-risk of ofatumumab remains unchanged. Patients should consult with their physicians and treatment decisions should be made based on individual patient benefit-risk assessment. Please refer to the Prescribing Information for information regarding the safety of ofatumumab
5. Should patients interrupt or discontinue therapy with ofatumumab if they are diagnosed with COVID-19?
- Limited data are available for providing any specific recommendations for people treated with ofatumumab
- Therapy with ofatumumab may need to be temporarily interrupted if a patient has a confirmed or suspected diagnosis of COVID-19 and/or severe symptoms; treatment can be restarted after COVID-19 resolution. Though the known benefit-risk of ofatumumab remains unchanged, physicians should make decisions based on individual patient benefit-risk assessment
- From the limited clinical study data, COVID-19 outcomes do not indicate a difference between ofatumumab-treated patients and the general population1
6. What is the guidance for ofatumumab treatment following a delay of a scheduled dose?
- Any delay in the scheduled ofatumumab administration is considered a ‘missed dose’. Please refer to your country national regulatory authority approved ofatumumab Prescribing Information for guidance on missed dose
- Per the country’s national regulatory authority approved ofatumumab Prescribing Information, if an injection of ofatumumab is missed, it should be administered as soon as possible without waiting until the next scheduled dose. Subsequent doses should be administered at the recommended intervals