Home > Key safety topics > Infections
Infection Rates in the MS Population
- Patients with MS are at an increased risk of infections compared with non-MS patients1,2
- The incidence rate of infections with monoclonal antibodies is comparable with that of the general MS population2
Overall Infections in Clinical Trials
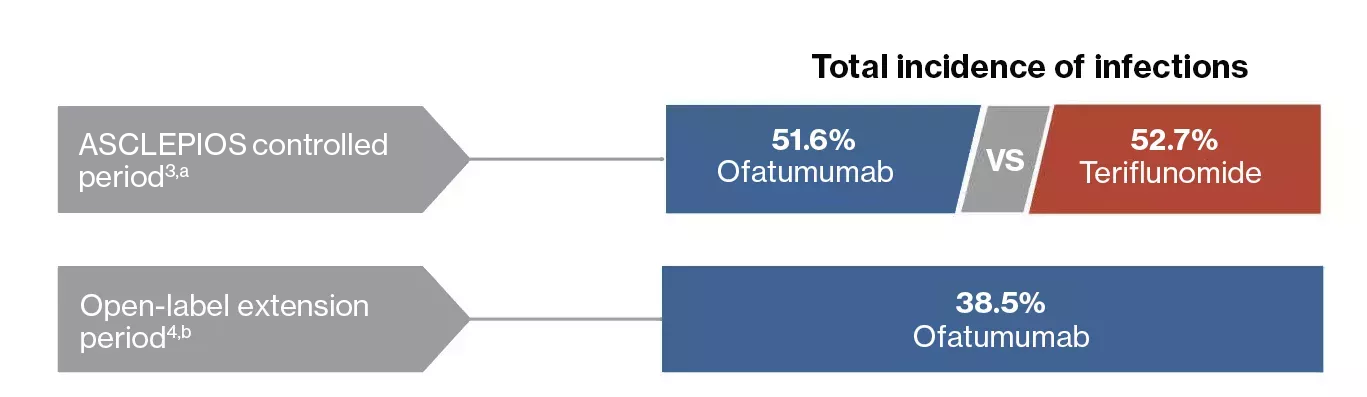
ASCLEPIOS controlled period3,a
- During the controlled period, overall incidence of infection was similar in the ofatumumab (51.6%) and teriflunomide (52.7%) groups
- Most common infections were upper respiratory tract and urinary tract infections
- Herpes viral infections were reported similar in ofatumumab (4.9%) and teriflunomide (4.2%) group. All herpes viral infections were non-serious, non-disseminated, resolved with treatment, and were not opportunistic
- Infections were mainly non-serious, Grade 1/2 severity, recovered with standard of care, and did not lead to study drug discontinuation or interruptions
Open-label extension period4,b
- As of 30 November 2019, no increase in the overall incidence of infections was observed in the ofatumumab-treated patients (38.5%) and were consistent with the controlled period (51.6%)
Serious Infections in Clinical Trials
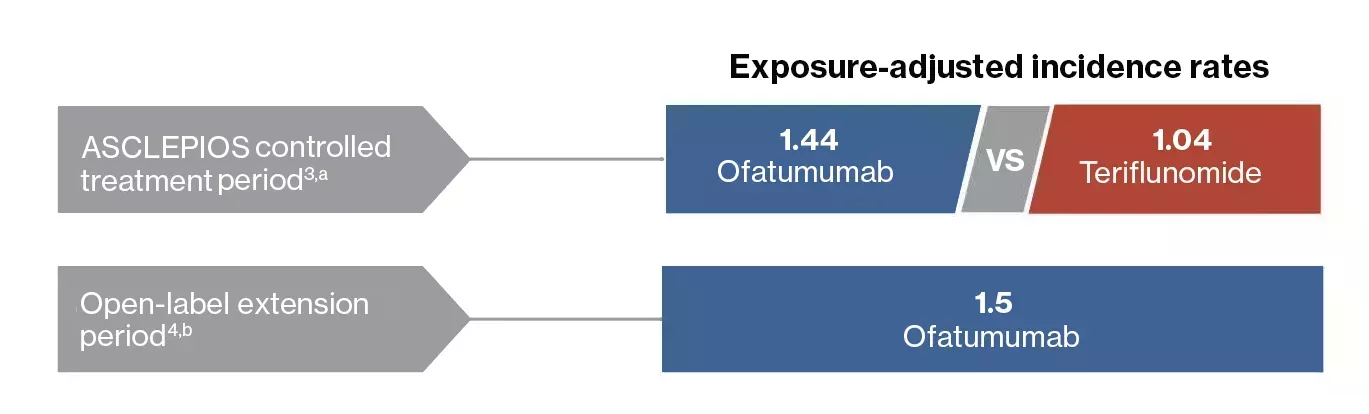
ASCLEPIOS controlled period3,a
- Overall, the incidence of serious infections was low in the ofatumumab and teriflunomide groups (2.5% and 1.8% of patients, respectively)
Open-label extension period4,b
- No increase in the incidence of serious infections was observed with ofatumumab (1.8% of patients)
Serious Opportunistic Infections in Clinical Trials
- No cases of serious opportunistic infectionshave been identified during the controlled period3,a or the open-label extension period4,b
- No cases of progressive multifocal leukoencephalopathy (PML) were reported in MS clinical studies with ofatumumab (see PML section for more details)
Serum Immunoglobulin Levels and Risk of Infections6,7
- Treatment effect of ofatumumab on IgG and IgM levels was analyzed for over a period of 3.5 years (Data cut-off:
29 January, 2021) in the following groups:- OMB overall pool (N=1969)c: Includes patients from long-term and teriflunomide/ofatumumab (TER/OMB) switch groups
- Long-term group (N=1292): Includes patients who received ofatumumab in the ASCLEPIOS I/II, APLIOS or APOLITOS core studies and continued with ofatumumab in the open-label extension. Patients who discontinued ofatumumab in core studies and did not enter their respective extension studies, but continued in the safety follow-up were also included
- TER/OMB switch group (N=677): Includes patients who received teriflunomide in the core part and switched to ofatumumab in the extension
- Over ~3.5 years of treatment with ofatumumab, serum immunoglobulin levels were consistent with the 96-week phase 3 ASCLEPIOS trial data, which showed that
- The mean IgG levels remained similar to baseline values and mean IgM levels remained above the LLN in the study
- IgG levels remained similar to the baseline values in all quartiles (Q1: 8.57, Q2: 10.07 and Q3: 11.51 g/L ) while, IgM levels decreased over time in all quartiles and the mean values remained above LLN
Serum IgG & IgM levels from baseline over time
Long-term OMB, N = 1292; TER/OMB, N = 677. For all pooled analyses, a fixed value of LLN (using ALITHIOS study reference) was used: IgG: 5.65 g/L; IgM: 0.4 g/L.
Ig, immunoglobulin; LLN, lower limit of normal; SE, standard error; RMS, relapsing multiple sclerosis; TER/OMB, switched from teriflunomide to ofatumumab.
- The overall incidence of serious infections in ofatumumab-treated patients was low (IR: 1.4 per 100 patient-years) for up to 3.5 years
- One patient in the IgG levels <LLN group and
3 patients in the IgM levels <LLN group reported serious infection
- One patient in the IgG levels <LLN group and
- There was no association between decreased Ig levels and the risk of serious infections
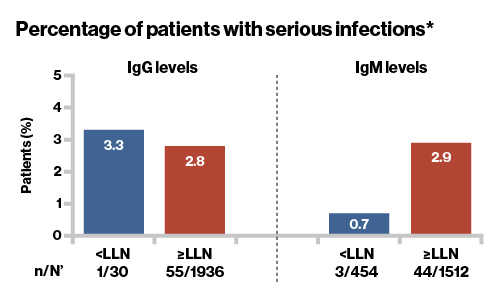
n, number of patients with infections
N', total number of patients with IgG/IgM levels below LLN at least once at any time during the post-baseline visits
*Patients with ≥1 SAE infection within 1 month prior and until 1 month after any series of drop in IgM/IgG levels <LLN; LLN, lower limit of normal
Patients with ≥1 serious infection within 1 month prior and until 1 month after any series of drops in IgM/IgG <LLN
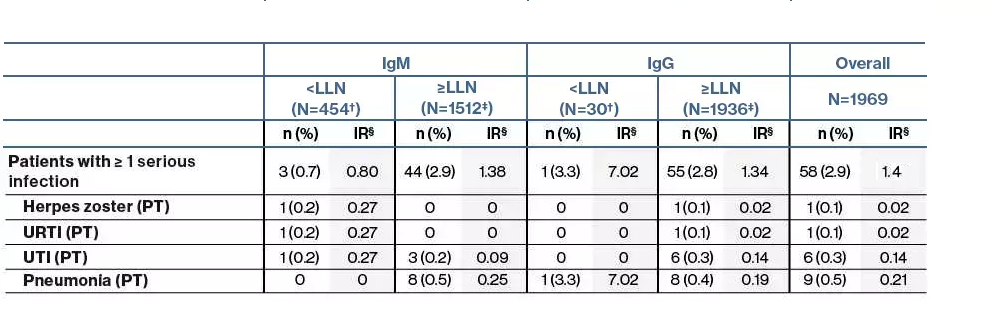
†Number of patients with IgM/IgG <LLN at least once at any time during the post-baseline visits; ‡Number of patients with no occurrence of IgM/IgG <LLN at least once at any time during the post-baseline visit; §IR per 100 PY estimated via a Poisson regression model with only treatment as the factor and with the log-link and natural logarithm of time as the offset variable. For all pooled analyses, a fixed value of LLN (using ALITHIOS study reference) was used: IgM: 0.4 g/L; IgG: 5.65 g/L
Ig, immunoglobulin; IR, incidence rate; LLN, lower limit of normal; PT, preferred term; PY, patient-year.

Additional information


