Home > Key safety topics > Malignancies
Malignancies in Clinical Trials
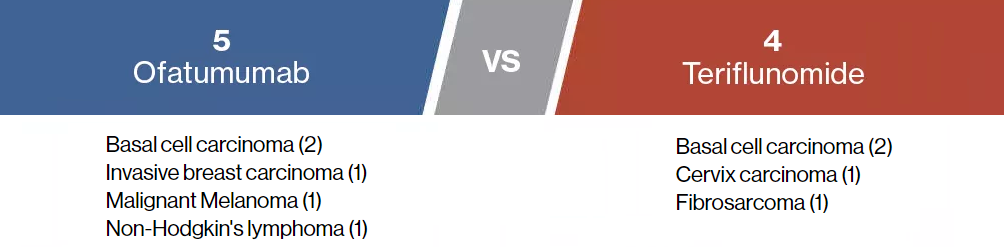
ASCLEPIOS controlled period1,a
- Five patients (0.5%) in the ofatumumab group reported malignant events compared to four patients in the teriflunomide group (0.4%)
- Of the five patients in the ofatumumab group:
- Invasive breast carcinoma was reported within 5 months of treatment initiation
- Malignant melanoma in situ was reported within 39 days
- Non-Hodgkin's lymphoma (recurrent) was diagnosed within 31 days
- The two cases of basal cell carcinoma were confounded and assessed by the investigator as not related to ofatumumab therapy
- The four cases of basal cell carcinoma (two in each treatment group) resolved after surgery, and patients were allowed to remain on study treatment after full excision of basal cell carcinoma
Open-label extension period2,b
-
No new cases of malignancies were observed