Infection Rates in the MS Population
- Patients with MS are at an increased risk of infections compared with non-MS patients1,2
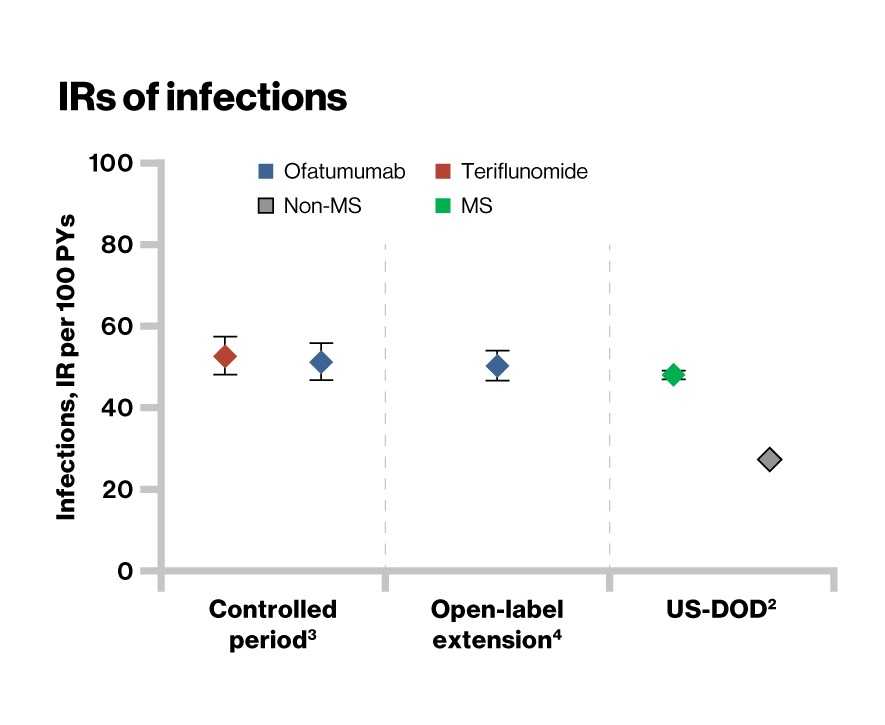
- The incidence rate of infections with monoclonal antibodies is comparable with that of the general MS population2
Overall Infections
Controlled period3,a
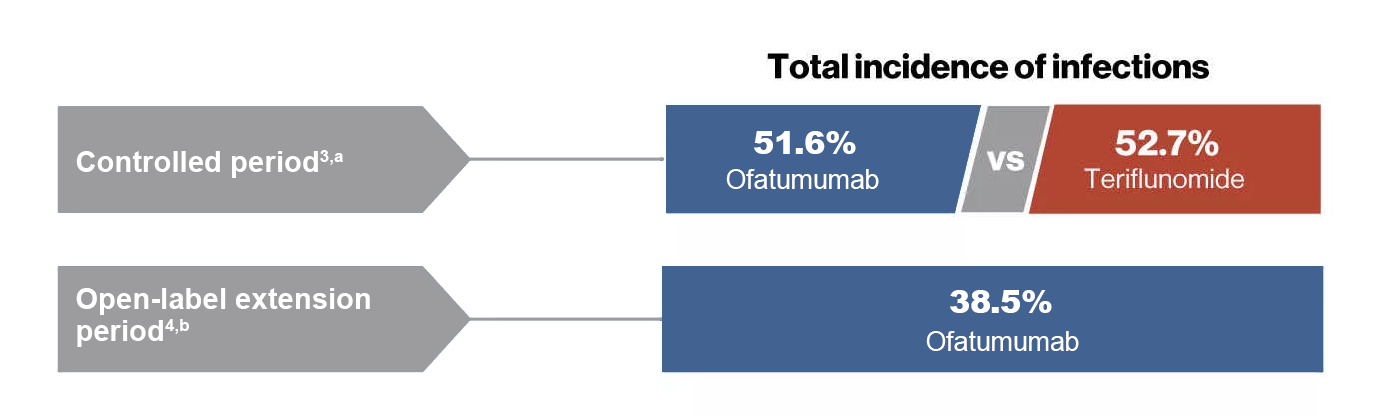
- During the controlled period, overall incidence of infection was similar in the ofatumumab (51.6%) and teriflunomide (52.7%) groups
- Most common infections were upper respiratory tract and urinary tract infections
- Herpes viral infections were reported similar in ofatumumab (4.9%) and teriflunomide (4.2%) group. All herpes viral infections were non-serious, non-disseminated, resolved with treatment, and were not opportunistic
- Infections were mainly non-serious, Grade 1/2 severity, recovered with standard of care, and did not lead to study drug discontinuation or interruptions
Open-label extension period4,b
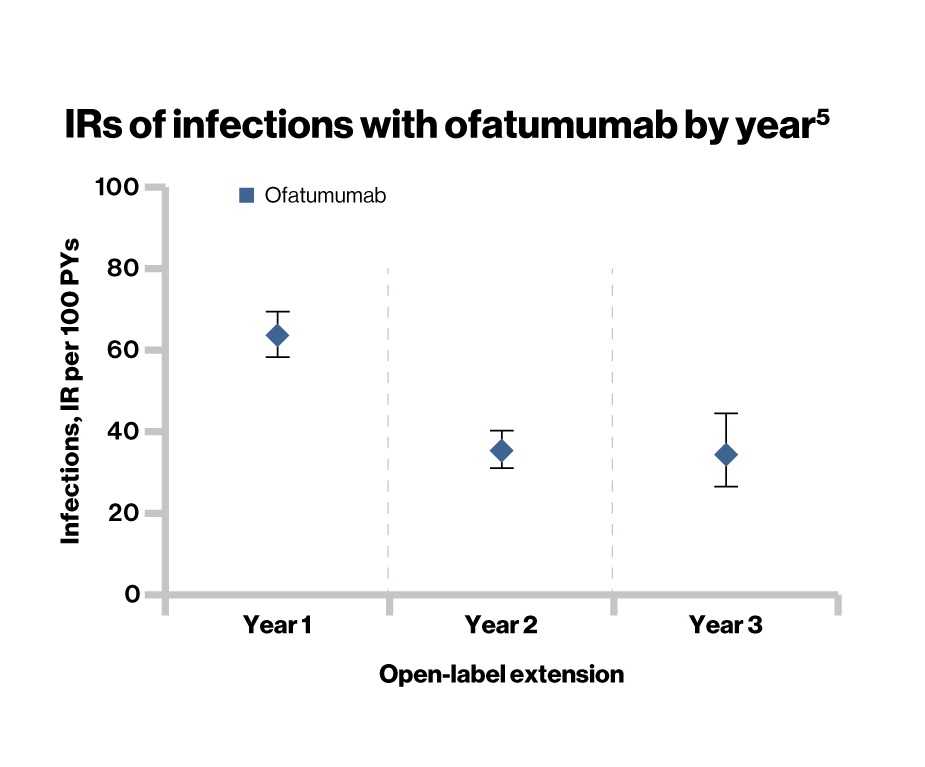
- As of November 30 2019, no increase in the overall incidence of infections was observed in the ofatumumab-treated patients (38.5%) and were consistent with the controlled period (51.6%)
Serious Infections
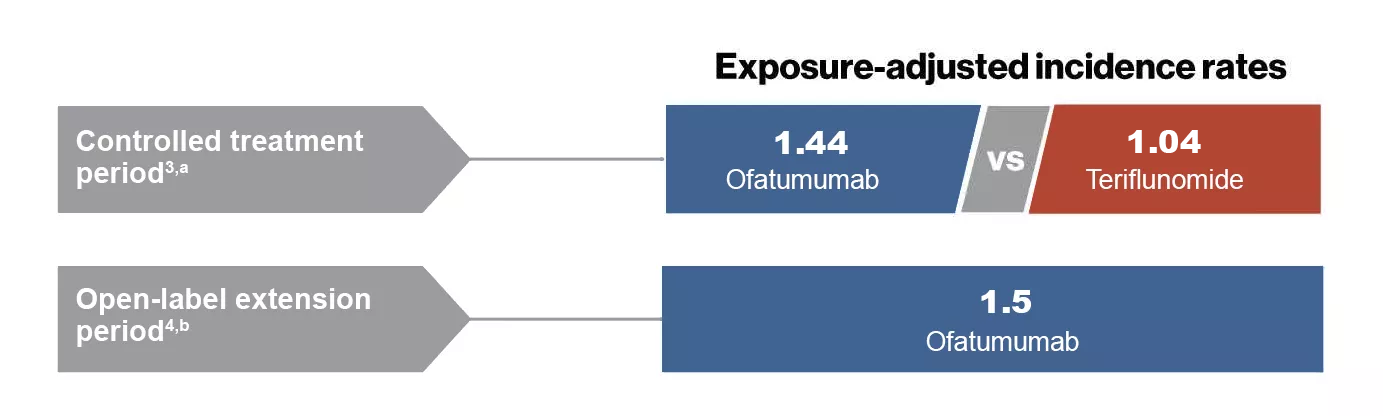
Controlled period3,a
- Overall, the incidence of serious infections was low in the ofatumumab and teriflunomide groups (2.5% and 1.8% of patients, respectively)
Open-label extension period4,b
- No increase in the incidence of serious infections was observed with ofatumumab (1.8% of patients)
Serious Opportunistic Infections
- No cases of serious opportunistic infectionshave been identified during the controlled period3,a or the open-label extension period4,b
- No cases of progressive multifocal leukoencephalopathy (PML) were reported in MS clinical studies with ofatumumab
(see PML section for more details)
Serum Immunoglobulin Levels and Risk of Infections6,7
- Treatment effect of ofatumumab on IgG and IgM levels was analyzed for over a period of 3.5 years (Data cut-off:
29 January, 2021) in the following groups:- Overall population (N=1969): Includes patients from long-term and TERIFLUNOMIDE/OFATUMUMAB (TER/OMB) switch groups
- Long-term group (N=1292): Includes patients who received ofatumumab in the ASCLEPIOS I/II, APLIOS or APOLITOS core studies and continued with ofatumumab in the open-label extension. Patients who discontinued ofatumumab in core studies and did not enter their respective extension studies, but continued in the safety follow-up were also included
- TER/OMB switch group (N=677): Includes patients who received teriflunomide in the core part and switched to ofatumumab in the extension
- Over ~3.5 years of treatment with ofatumumab, serum immunoglobulin levels were consistent with the 96-week phase 3 ASCLEPIOS trial data8, which showed that
- The mean IgG levels remained similar to baseline values and mean IgM levels remained above the LLN in the study
- IgG levels remained similar to the baseline values in all quartiles (Q1: 8.57, Q2: 10.07 and Q3: 11.51 g/L while, IgM levels decreased over time in all quartiles and maximum slope was observed in patients with highest quartile group (Q1, 0.81; Q2, 1.14; Q3, 1.57 )
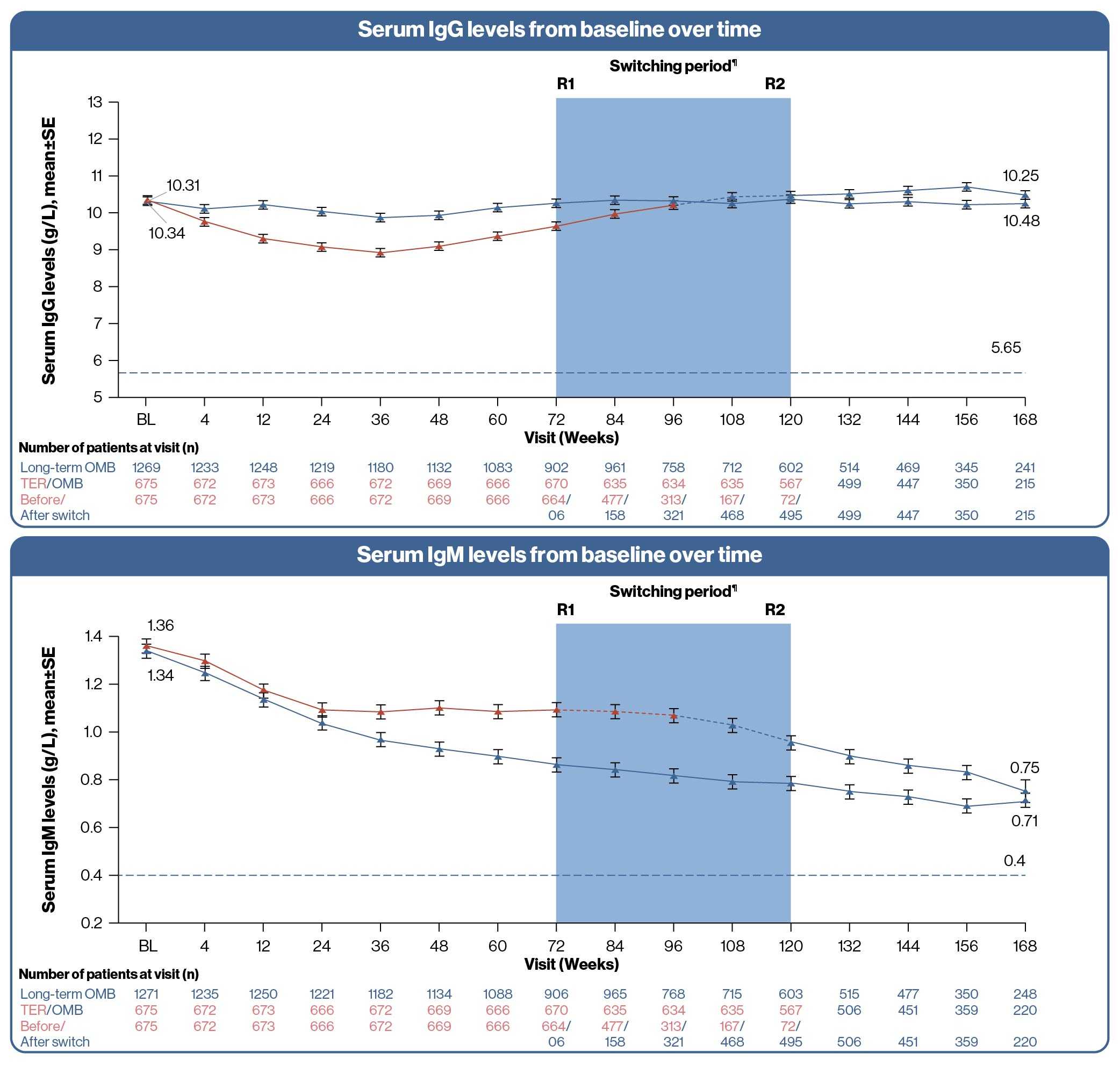
Long-term OMB, N=1292; TER/OMB, N=677. For all pooled analyses, a fixed value of LLN (using ALITHIOS study reference) was used: IgG: 5.65 g/L; IgM: 0.4 g/L
R1: The first patient with first treatment emergent assessment in OMB period after switching to OMB (72 weeks)
R2: The last patient with last treatment emergent assessment in TER period before switching to OMB (120 weeks)
¶Switching period refers to the patients started with teriflunomide and not applicable to the patients with ofatumumab in core period; For TER/OMB group, data from the first dose of TER till last dose of OMB plus 100 days/analysis cutoff date have been used
Ig, immunoglobulin; LLN, lower limit of normal; SE, standard error; RMS, relapsing multiple sclerosis; TER/OMB, switched from teriflunomide to ofatumumab
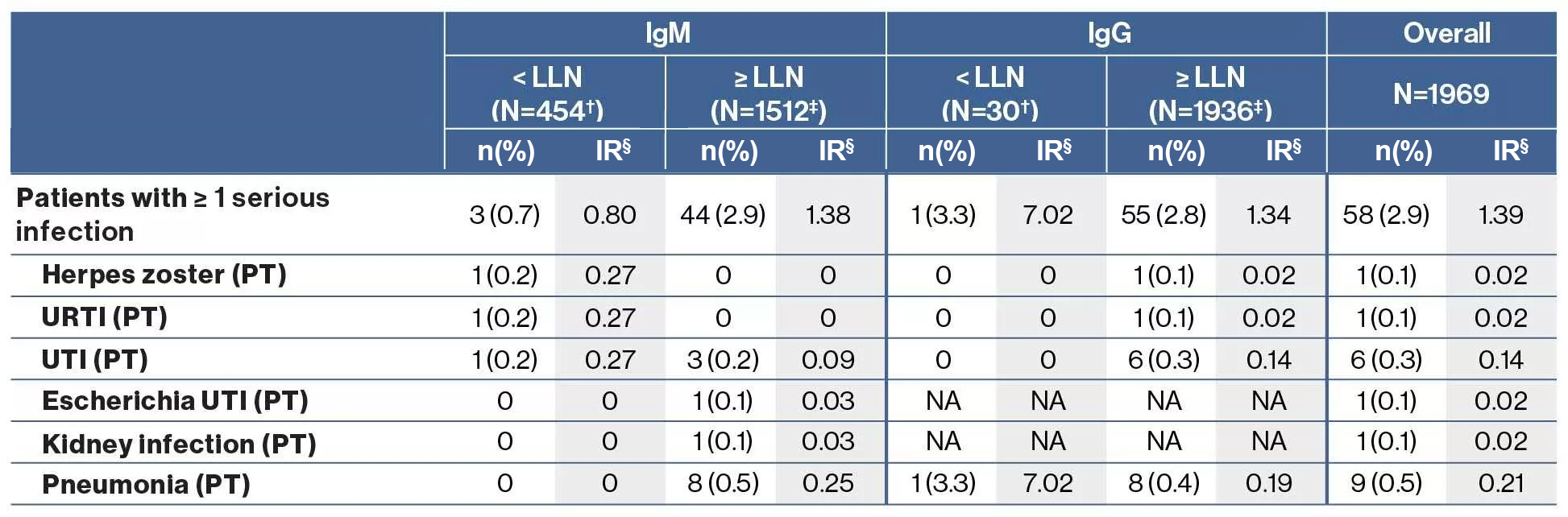
- The overall incidence of serious infections in ofatumumab-treated patients was low (IR: 1.39 per 100 patient-years) for up to 3.5 years
- One patient in the IgG levels <LLN group and
3 patients in the IgM levels <LLN group reported serious infection
- One patient in the IgG levels <LLN group and
- There was no association between decreased Ig levels and the risk of serious infections
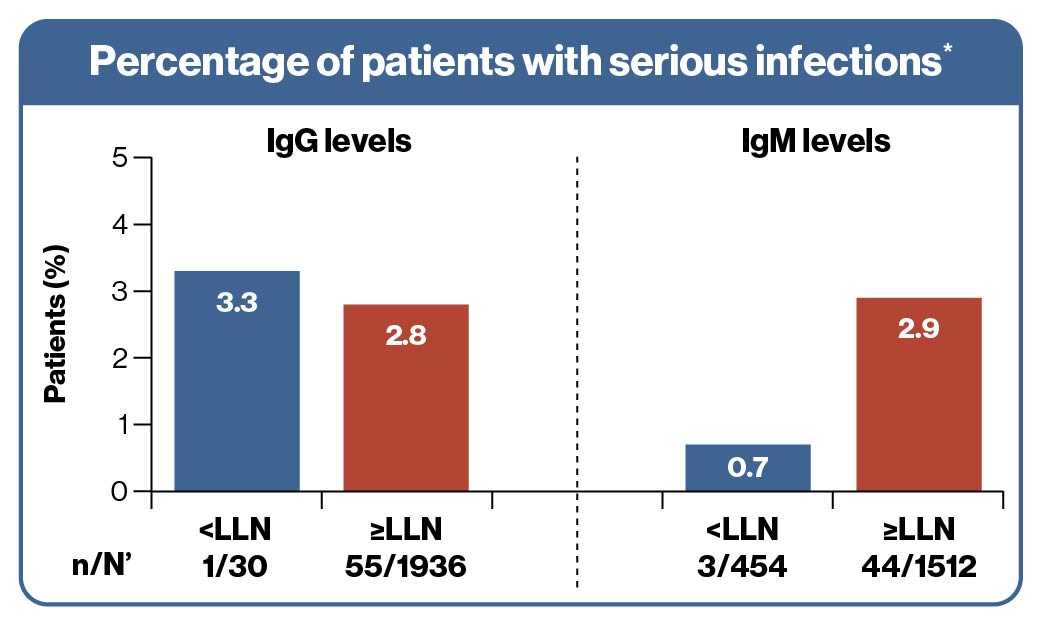

Patients with ≥1 serious infection within 1 month prior and until 1 month after any series of drops in IgM/IgG <LLN