Our commitment to
ongoing communication
on safety
A verified up-to-date global source of safety
information from clinical trials and
post-marketing experience with
ofatumumab for Healthcare Professionals
Website full content update:
July 2022
Latest Updates: November 2022
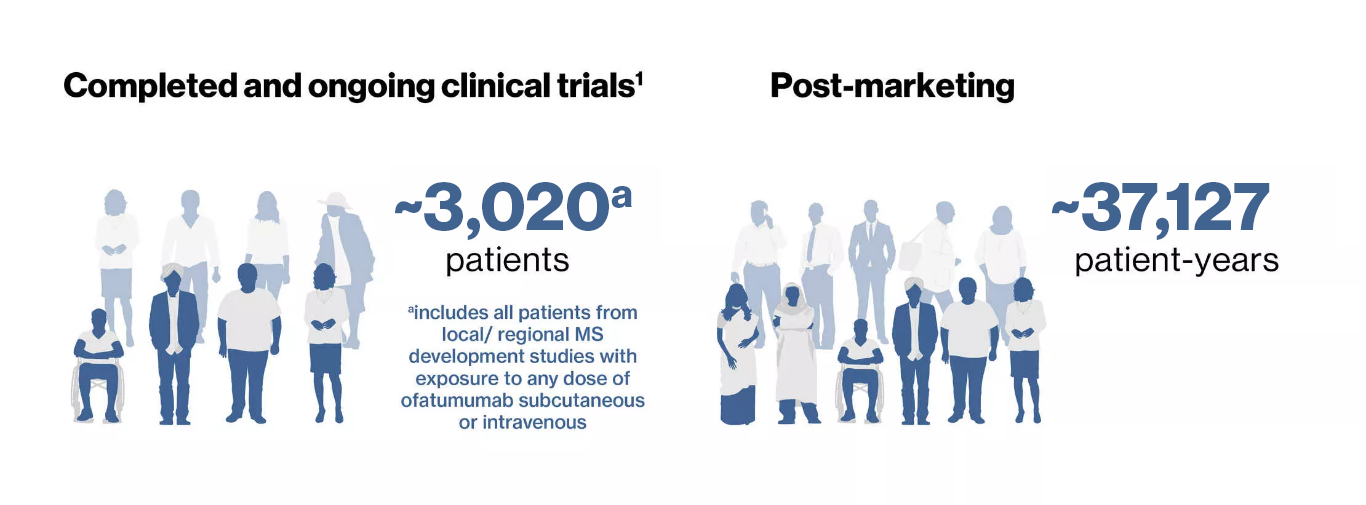
As of 25 Sep 2022, a total of approximately 3,020 patients with relapsing multiple sclerosis (RMS) received ofatumumab in clinical trials including the ongoing open-label extension and there was approximately 37,127 patient-years post marketing experience1