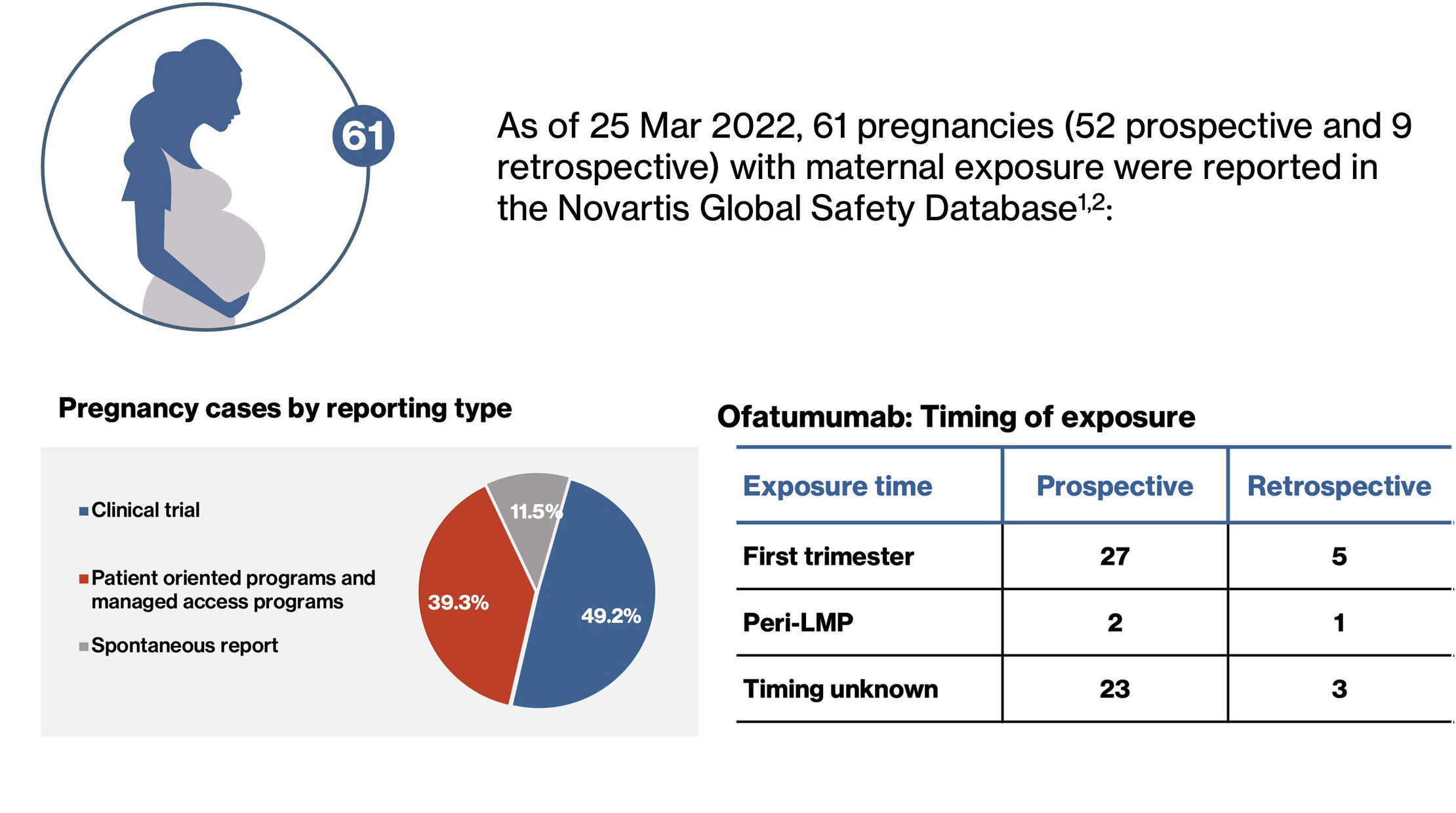
- Ofatumumab treatment was discontinued in all women following confirmation of the pregnancy
Pregnancy outcomes
As of the cut-off date, outcomes were known in 30 pregnancies:
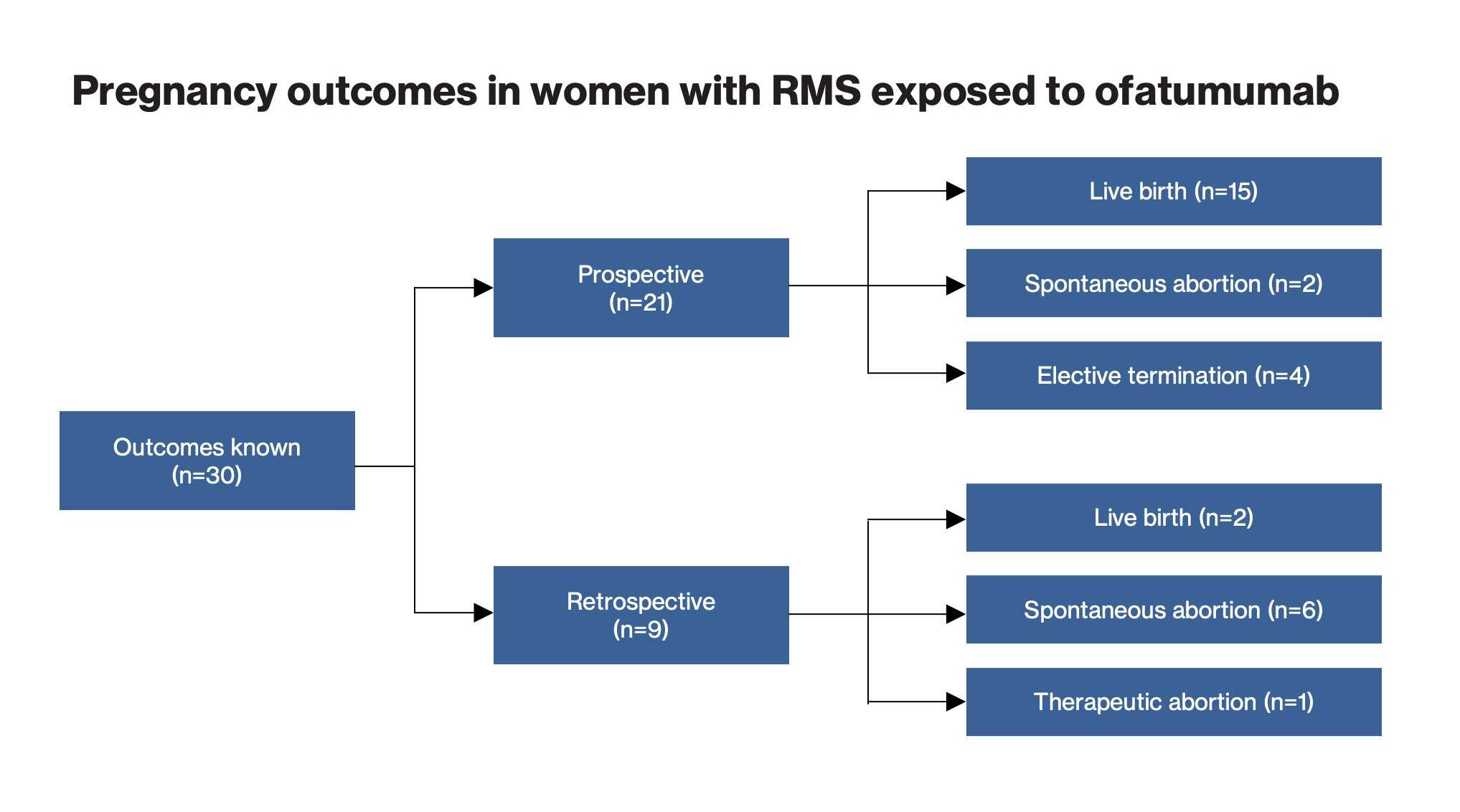
Infant outcomes

Ofatumumab may cause fetal harm based on animal data. Advise females of reproductive potential of the potential risk to a fetus and to use an effective method of contraception during treatment and for 6 months after stopping ofatumumab. For more details, please refer to the Prescribing Information.
aanomalies corresponds to both during pregnancy or post-partum.
Additional information


References
1. Data on File. PSUR (Cut-off date: 25 Mar 2022); Novartis Pharma AG.
2. Hellwig K, et al. Poster presented at CMSC 2022.